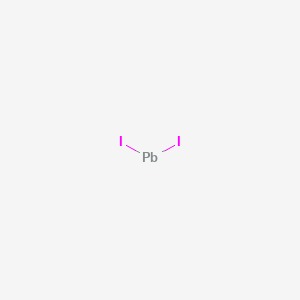Lead iodide is a chemical compound with the formula PbI2. It is a yellow solid that is highly insoluble in water, but readily dissolves in organic solvents such as ethanol and acetone. Lead iodide is typically synthesized by the reaction of lead metal with iodine gas, which produces lead iodide and lead monoxide as byproducts. The reaction can be represented by the following balanced chemical equation:
2 Pb + I2 -> 2 PbI2 + O2
Lead iodide has several interesting properties that make it useful in a variety of applications. For example, it is a highly efficient absorber of light, making it an excellent candidate for use in solar cells. It is also an effective conductor of electricity, making it useful in the production of electronic devices.
One of the most well-known uses of lead iodide is in the production of x-ray film. When x-rays pass through an object, they interact with the lead iodide in the film, producing an image of the object on the film. This image can then be used to diagnose medical conditions or to inspect the structure of materials in various industries.
In addition to its practical uses, lead iodide has also been the subject of scientific research. Scientists have studied its crystal structure and have found that it exhibits unusual behavior when subjected to high pressures and temperatures. This has led to the discovery of several new materials with unique properties, which could have potential applications in a variety of fields.
Despite its many useful properties, lead iodide can also be harmful to humans if ingested or inhaled. It is classified as a toxic substance, and appropriate precautions should be taken when handling it.
In summary, lead iodide is a chemical compound with the formula PbI2 that has a variety of practical and scientific applications. It is an excellent absorber of light, a good conductor of electricity, and is useful in the production of x-ray film. However, it is also toxic and should be handled with care.