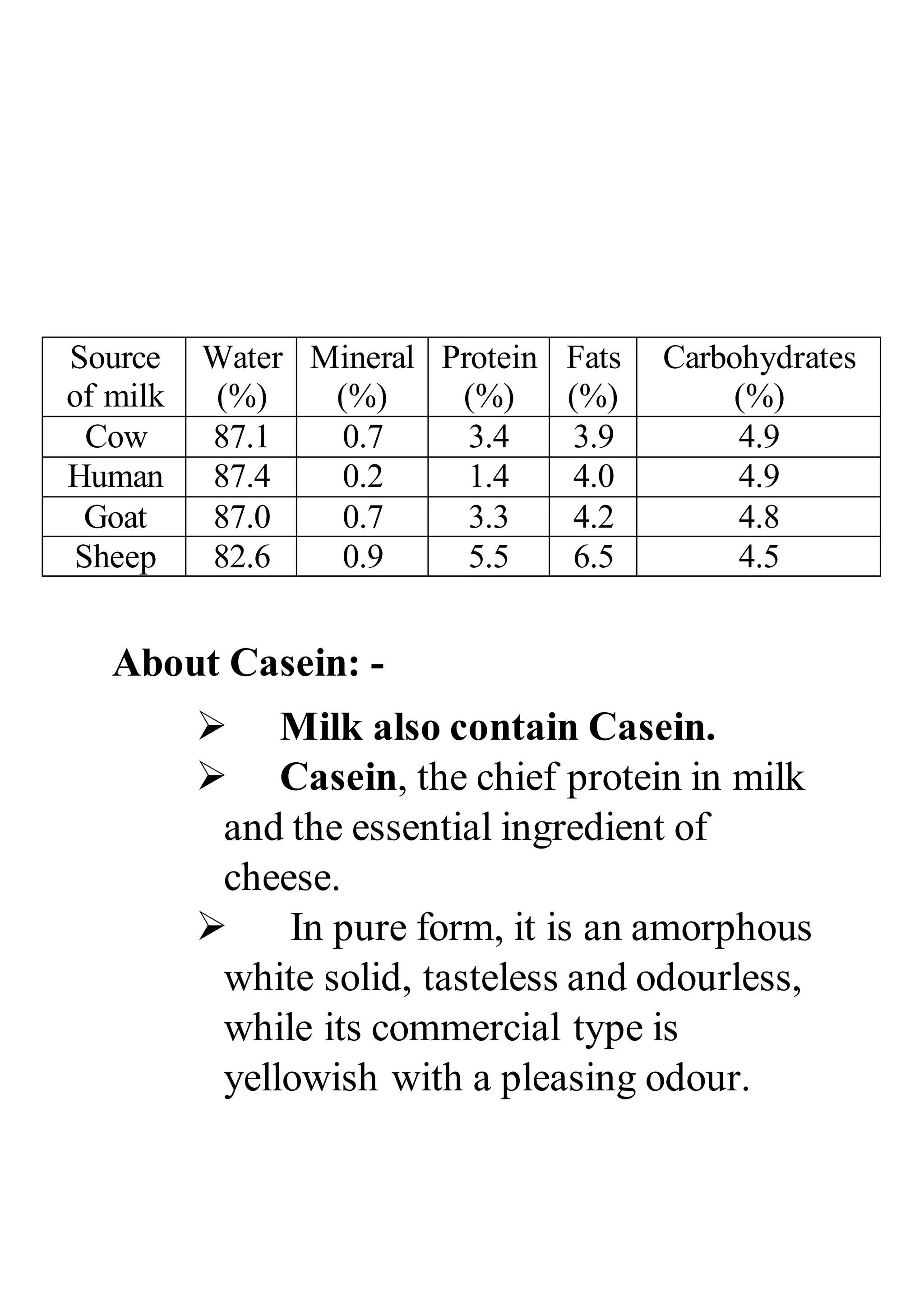
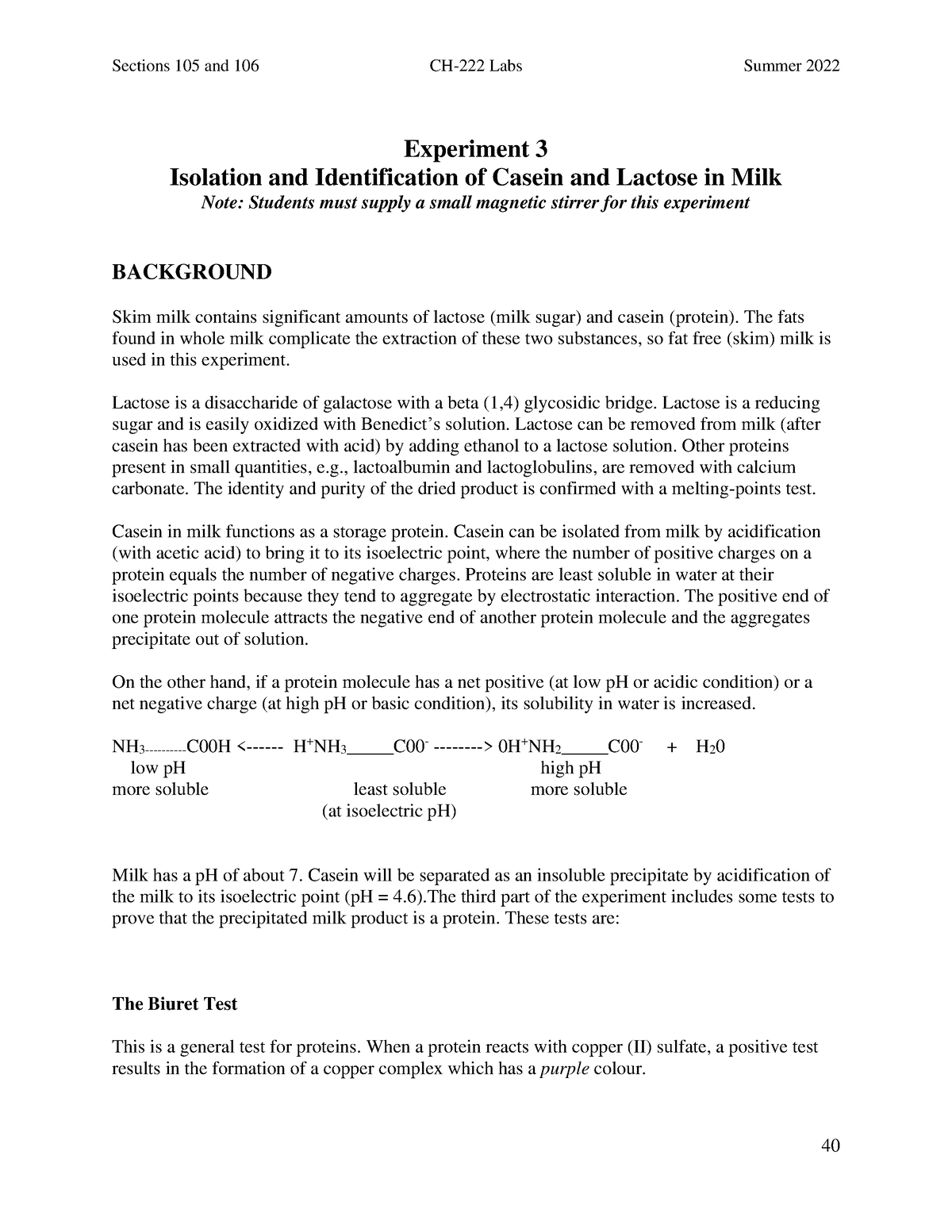
Casein is a group of phosphoproteins that are found in the milk of mammals. It makes up approximately 80% of the protein content of cow's milk and plays a vital role in the nutrition of infants. In addition to its nutritional value, casein has many industrial uses, including the production of cheese, yogurt, and other dairy products. It is also used as a binder in the production of paints, plastics, and adhesives.
One method of isolating casein from milk is through the process of acid precipitation. This involves adding an acid, such as hydrochloric acid, to milk, which causes the casein to coagulate and separate from the other proteins and fats in the milk. The resulting curd can then be collected and washed to remove any remaining impurities.
Another method of isolating casein from milk is through the use of rennet, which is an enzyme that is naturally present in the stomachs of mammals. When added to milk, rennet causes the casein to coagulate, forming a solid curd that can be collected and washed.
In both of these methods, the resulting casein can be dried and ground into a powder, which can be used in a variety of applications.
In addition to these traditional methods, casein can also be isolated using more modern techniques, such as chromatography or centrifugation. These methods allow for the separation of specific proteins within the milk, allowing for the isolation of purer forms of casein.
Regardless of the method used, the isolation of casein from milk is an important process that is used in a variety of industries. It allows for the production of a wide range of dairy products, as well as the utilization of casein in other applications, such as the production of paints, plastics, and adhesives.