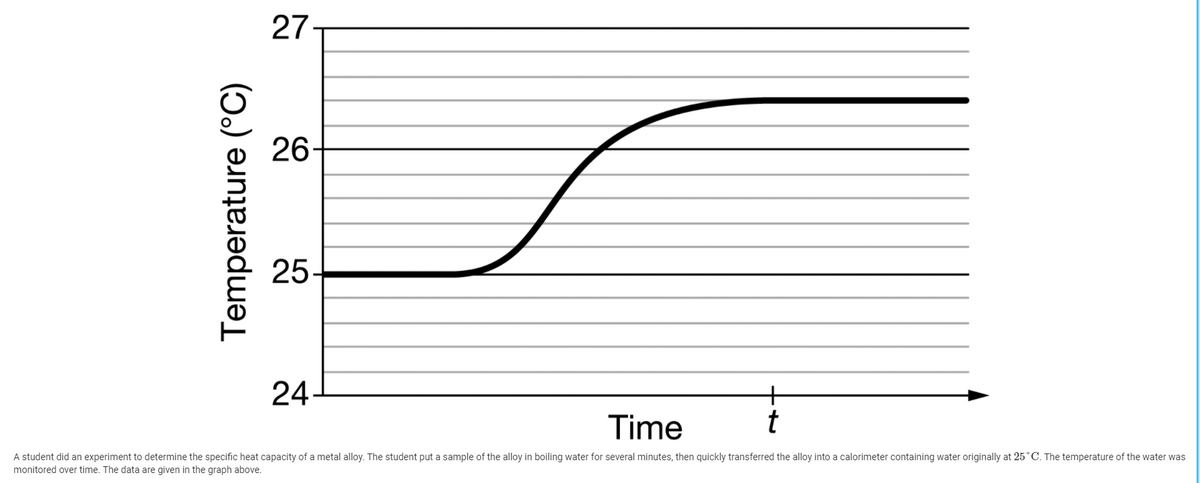
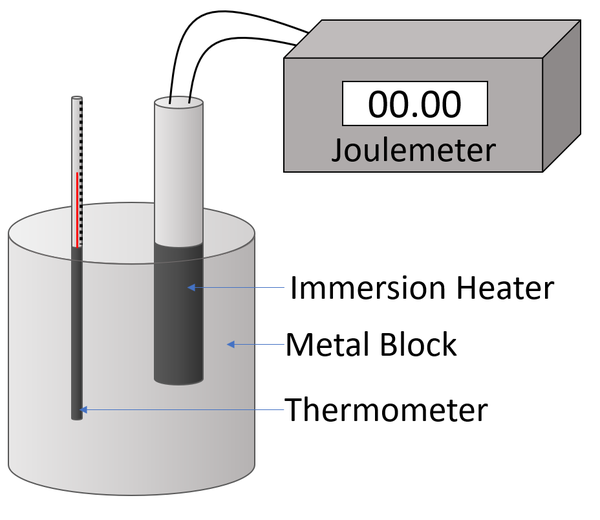
Specific heat capacity is a measure of the amount of energy required to raise the temperature of a substance by a certain amount. It is an important property of materials, particularly in the field of thermodynamics, as it determines the amount of energy needed to perform various thermal processes. In this essay, we will discuss how to calculate the specific heat capacity of a metal.
There are several methods for calculating the specific heat capacity of a metal, but one common method is known as the "heat capacity method." This method involves heating a known mass of the metal to a known temperature, and then measuring the amount of heat required to raise the temperature of the metal by a certain amount.
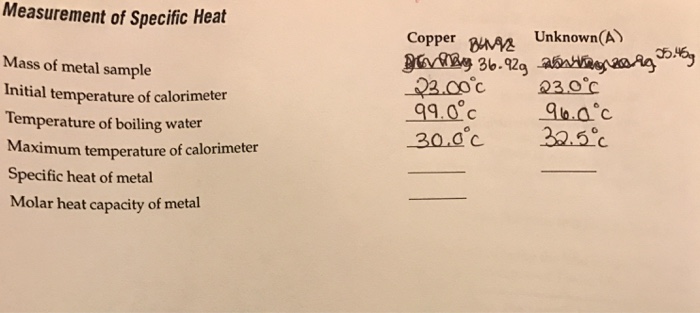
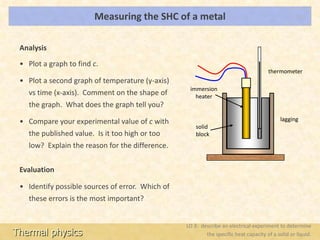
To begin, you will need to gather the following items: a metal sample, a calorimeter (a device used to measure heat transfer), a thermometer, and a heat source.
First, place the metal sample in the calorimeter and measure its initial temperature using the thermometer. Next, add a known amount of heat to the metal using the heat source. It is important to measure the amount of heat added accurately, as this will affect the accuracy of the specific heat capacity calculation.
After the heat has been added, measure the final temperature of the metal using the thermometer. The difference between the initial and final temperatures is known as the temperature change, or ΔT.
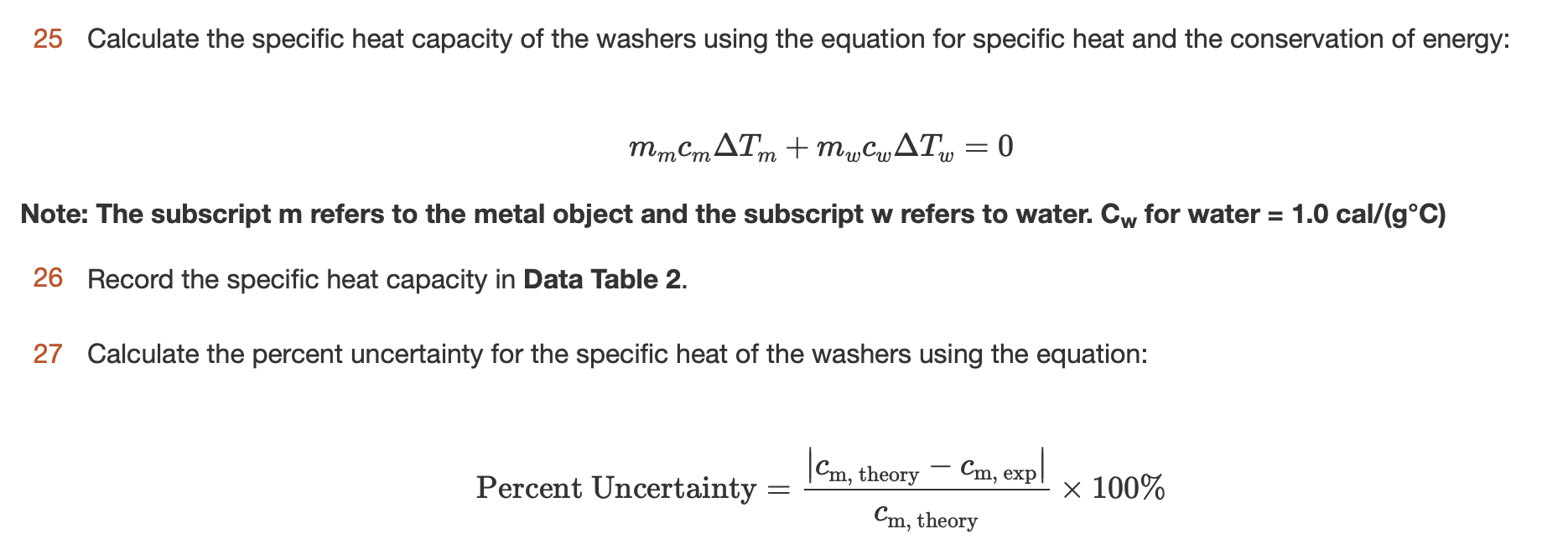
Now that you have gathered all of the necessary data, you can calculate the specific heat capacity of the metal using the following equation:
Specific heat capacity = (Heat added) / (Mass of metal x Temperature change)
The units of specific heat capacity are typically given in joules per gram-kelvin (J/g-K).
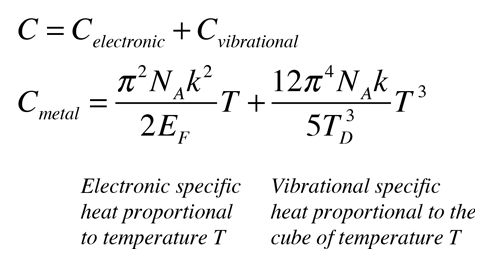
It is important to note that the specific heat capacity of a metal may vary depending on its temperature and the conditions under which it is measured. For example, the specific heat capacity of a metal may be different at high temperatures compared to low temperatures.
In conclusion, the specific heat capacity of a metal can be calculated using the heat capacity method, which involves heating a known mass of the metal to a known temperature and measuring the amount of heat required to raise the temperature of the metal by a certain amount. This important property of materials is used in a variety of applications, including thermodynamics and heat transfer.