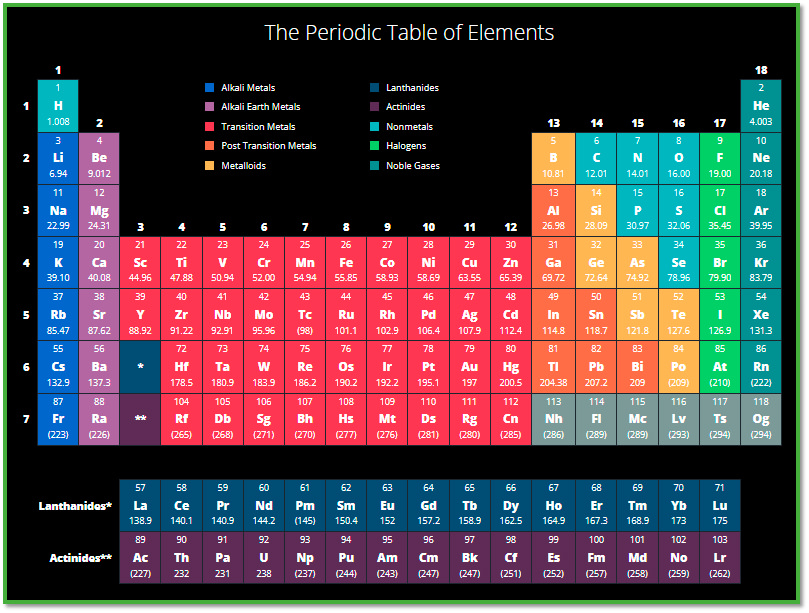
Henry Moseley was a British physicist who made significant contributions to the understanding of the periodic table of elements. Moseley is best known for his work on the concept of atomic number, which he introduced in 1913.
At the time, the periodic table was based on the atomic mass of each element, and there were several discrepancies that could not be explained. Moseley proposed that the properties of an element were not determined by its atomic mass, but rather by its atomic number, which is the number of protons in the nucleus of an atom. This revolutionary idea allowed for a more accurate and consistent organization of the periodic table.
Moseley used X-ray spectra to determine the atomic number of each element. He observed that the X-ray spectra of different elements had unique patterns, which he attributed to the differences in the number of protons in the nuclei of their atoms. By comparing the spectra of different elements, Moseley was able to assign an atomic number to each element and create a more accurate periodic table.
Moseley's work on the periodic table had far-reaching implications for the field of chemistry. It provided a more precise method for predicting the properties of elements, which led to a better understanding of their chemical behavior. It also provided a foundation for the development of the modern model of the atom, which is based on the idea that the properties of an atom are determined by its atomic number.
In addition to his work on the periodic table, Moseley also made important contributions to the understanding of X-ray spectra and the nature of the atom. He is considered one of the pioneers of modern physics and his work has had a lasting impact on the field.
In conclusion, Henry Moseley was a groundbreaking physicist whose work on the periodic table of elements helped to revolutionize our understanding of the atom and the nature of matter. His contributions have had a lasting impact on the field of chemistry and continue to be recognized and celebrated today.
What is Moseley's Periodic Table?

The properties of elements were repeating after regular intervals. Soon Moseley, who had always had a keen interest in chemistry, began to examine how a sequence of elements following each other in the periodic table might behave when acting as targets for beams of X-rays. Consultation from Neils Bohr and Braggs proved to be a big help for him in performing this experiment. Explanation of 7th point Concept: Valence electrons are the number of electrons present in the outermost orbit of an atom. Philosophical Transactions of the Royal Society A: Mathematical, Physical, and Engineering Sciences, March 13, 2015: United Nations Educational, Scientific, and Cultural Organization.
Moseley's Periodic Table

Moseley quickly got a research fellowship and concentrated entirely on research, quitting the teaching part of his work. The former use of atomic weights to order the elements suggested that one or perhaps two elements might be missing between hydrogen and helium, the two lightest known elements. Moseley discovered a correlation between the number of protons in atoms, their atomic number, and the X-rays they produced. However, it was soon discovered that arranging elements in this manner did not correlate with the position predicted by their chemical properties. Introduction The International Year of the Periodic Table 2019 marked the sesquicentenary of the publication by Dmitri Mendeleev in 1869 of his first version of a periodic table of the elements, ordered in rows and columns on the basis of their atomic weights, now given the symbol A. His work with x-ray spectroscopy, in which electrons are fired at a metal plate, causing the atoms of metal to emit x-rays, allowed us to accurately measure the atomic number , which tells us the number of protons in an atom of any given element, of each element.
How did Moseley contribute to the periodic table?
All you know about the elements identified at the time is how they interact with each other, their physical properties, and their relative atomic weights. At Eton his first passion was chemistry and his award of a Millard Scholarship at Trinity College Oxford starting in 1906 was probably made on the basis that he would read this subject for his final degree. Leave a comment on 61. Time to break out the helium balloons, iron-based sparklers, and calcium-rich ice cream! He was killed on 10 August 1915 by a bullet to the head at the battle of Gallipoli in Turkey. For chemists, the number of those acidic hydrogen ions can be quantified by using the pH scale. In x-ray spectroscopy, electrons are fired at a metal plate, causing the atoms of metal to emit x-rays. His full name was Henry Gwyn Jeffreys Mosely.
Understanding Henry Moseley’s Periodic Table and His Life

His paternal grandfather had been a … So must visit that article. Biography of Henry Moseley Henry Moseley, called Harry by family and friends, was born on November 23, 1887 in Dorset, England. From there he moved on to Trinity College in Oxford, where he received high academic accolades. This discovery is now known as Moseley's Law. He used the technique of x-ray spectroscopy. Don't miss the latest corrosion content from Corrosionpedia! Iodine has a lower relative atomic mass than tellurium.