Gravimetric analysis is a type of quantitative analytical method that involves the measurement of the mass of a substance in order to determine its concentration or purity. In the case of a chloride salt, such as sodium chloride or potassium chloride, gravimetric analysis can be used to determine the amount of chloride present in a sample.
There are several different approaches to conducting a gravimetric analysis of a chloride salt, but the most common method involves precipitation. Precipitation is the process of forming a solid from a solution by adding a reagent that reacts with the dissolved substance to produce a solid precipitate.
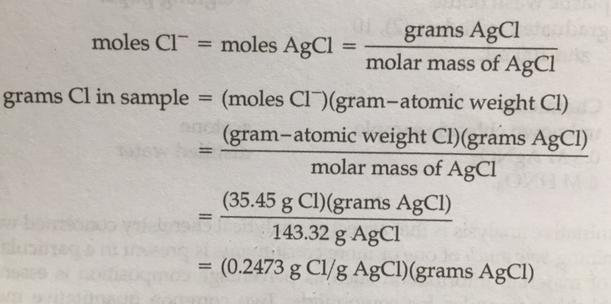
To begin the analysis, a sample of the chloride salt is dissolved in water to form a solution. Next, a reagent is added to the solution that reacts with the chloride ions to produce a solid precipitate. The most common reagent for this purpose is silver nitrate, which reacts with chloride ions to form silver chloride.
The precipitate is then allowed to settle to the bottom of the container, and the solution is decanted or filtered off. The precipitate is then dried and weighed to determine the mass of the chloride present in the sample.
The mass of the chloride in the sample can then be used to calculate the concentration of chloride in the solution. This can be done by comparing the mass of the chloride in the sample to the mass of the entire sample, or by using a standard curve.
In order to obtain accurate results, it is important to carefully control the conditions of the reaction and to carefully clean and dry the precipitate before weighing it. It is also important to use a high-quality balance or scale to accurately measure the mass of the precipitate.
Overall, gravimetric analysis is a useful and reliable method for determining the concentration of a chloride salt in a sample. By carefully controlling the conditions of the reaction and accurately measuring the mass of the precipitate, it is possible to obtain precise and accurate results.
Gravimetric Analysis of Chloride Salt Lab Report

Volumetric analysis, on, the other hand, derives its name from the fact that the method used to determine the amount of a constituent involves measuring the volume of a reagent. PURPOSE Using variable laboratory techniques, a quantitative analysis of the content of chloride salt was determined in an unknown salt content. Why we heat to coagulate. Gravimetric analysis derives its name from the fact that the constituent being determined can be isolated in some weighable form. Observations Observation Description Physical description of sample Sample number:343 Sample had a light appearance, white. The crucible was washed once again in the vacuum filtration arrangement. Barium can be analyzed by precipitating it as BaSO4 and weighing the precipitate.
Gravimetric Analysis of A Chloride Salt

The nature of the contents will be determined by the practicability of the methods in undergraduate teaching, by their acceptability for research publications, and by their affordability by public sector institutions. The uncertainties for sample 1 and 2 were ±0% and ±0%, respectively. Anions from some acids may co-precipitate with the chloride ion, forming co precipitates. Even though she may not have lost a lot of her sample, her initial salt mass was just 1. The vacuum filtration arrangement was set up. The acetone was handed to the T. After a couple washings the precipitate was also placed into the filter.
Gravimetric Analysis of a Chloride Salt

The heated liquid was decanted through the funnel and a few mL of 0 HNO 3 was added to precipitate in the beaker and the washing was repeated until the beaker was empty of all liquids and solids. This process allows it to become more difficult for the precipitate to go through the filter. Find the mean, the standard deviation, and the relative standard deviation. Prac Report-Precipitation It is expected that the following compounds will form precipitates Silver Sulphate, Copper Hydroxide, Copper Iodide, Silver Hydroxide, Silver Iodide, Cobalt Hydroxide and two Silver Chlorides. This produces a lower result for the chloride determination but when the experiment is carried out, the effect is not as much if it is not exposed to direct sunlight for a prolonged time.