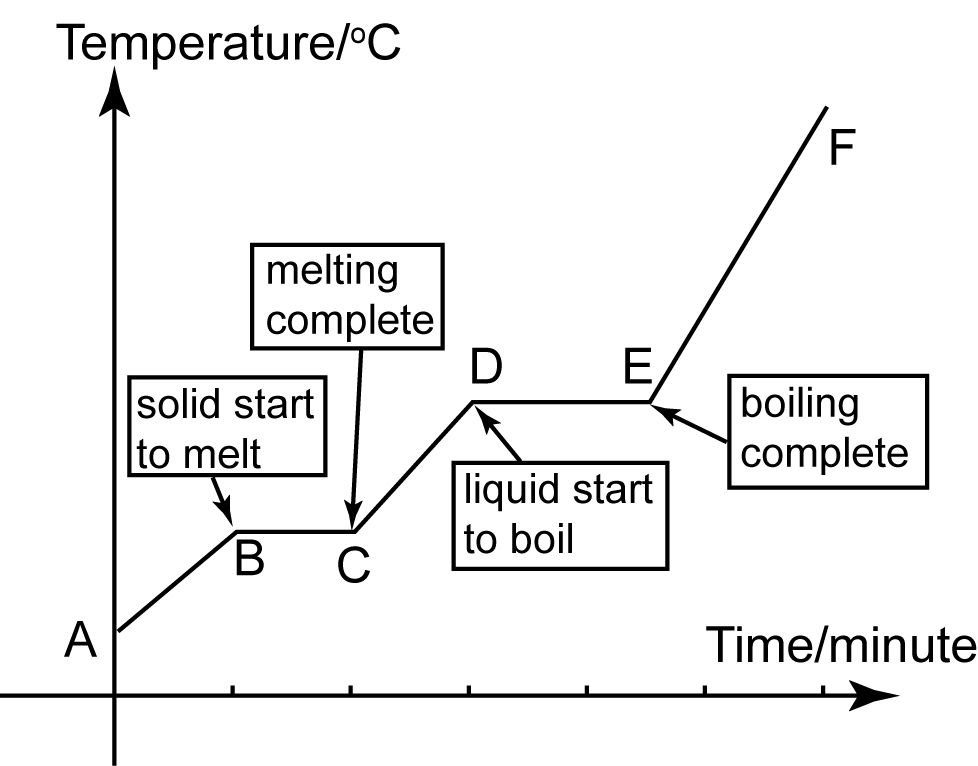
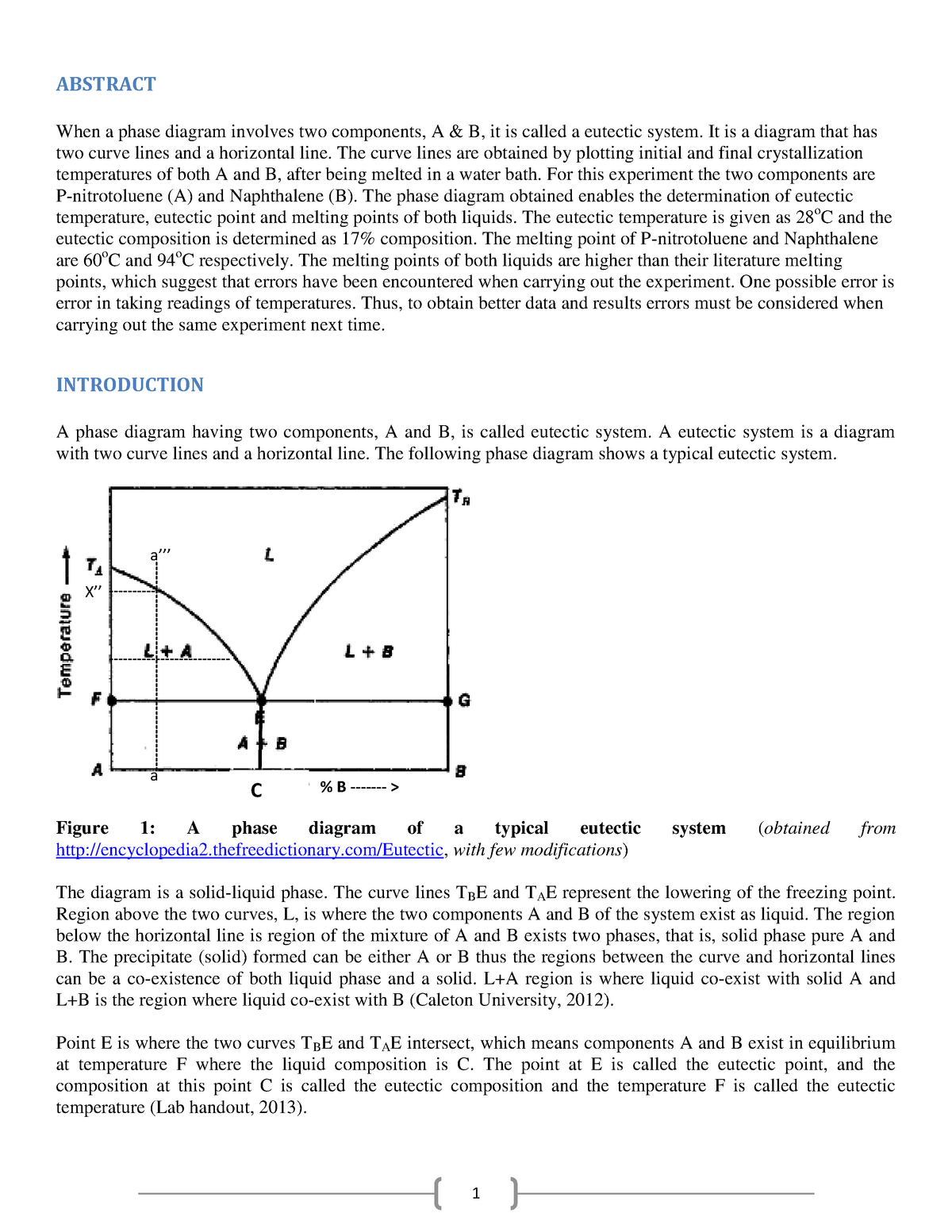
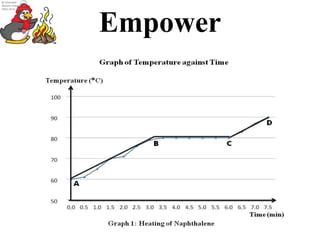
The freezing point of a substance is the temperature at which it changes from a liquid to a solid. The freezing point of naphthalene, also known as mothballs, can be represented on a graph as a horizontal line at a specific temperature.
To construct a graph of the freezing point of naphthalene, one would need to perform a series of experiments to determine the temperature at which naphthalene changes from a liquid to a solid. This can be done by placing a sample of naphthalene in a container with a thermometer and gradually decreasing the temperature until the naphthalene begins to solidify. The temperature at which this occurs can then be recorded and plotted on the graph as a horizontal line.
One interesting aspect of the freezing point of naphthalene is that it is not a constant value, but rather it is dependent on the pressure at which the naphthalene is being held. At high pressures, the freezing point of naphthalene will be higher than at low pressures. This relationship can also be represented on a graph, with the freezing point plotted as a function of pressure.
In addition to the freezing point, other physical properties of naphthalene such as its melting point, boiling point, and vapor pressure can also be plotted on a graph. These properties can provide valuable information about the behavior of naphthalene under different conditions, and can be used to predict how it will behave in a variety of applications.
Overall, the freezing point of naphthalene can be represented on a graph as a horizontal line at a specific temperature, and this line can be plotted as a function of pressure to show the relationship between the freezing point and pressure. Understanding the physical properties of naphthalene can be useful for a variety of applications, from pest control to industrial uses.
Determining the Freezing Point of Pure webapi.bu.edu
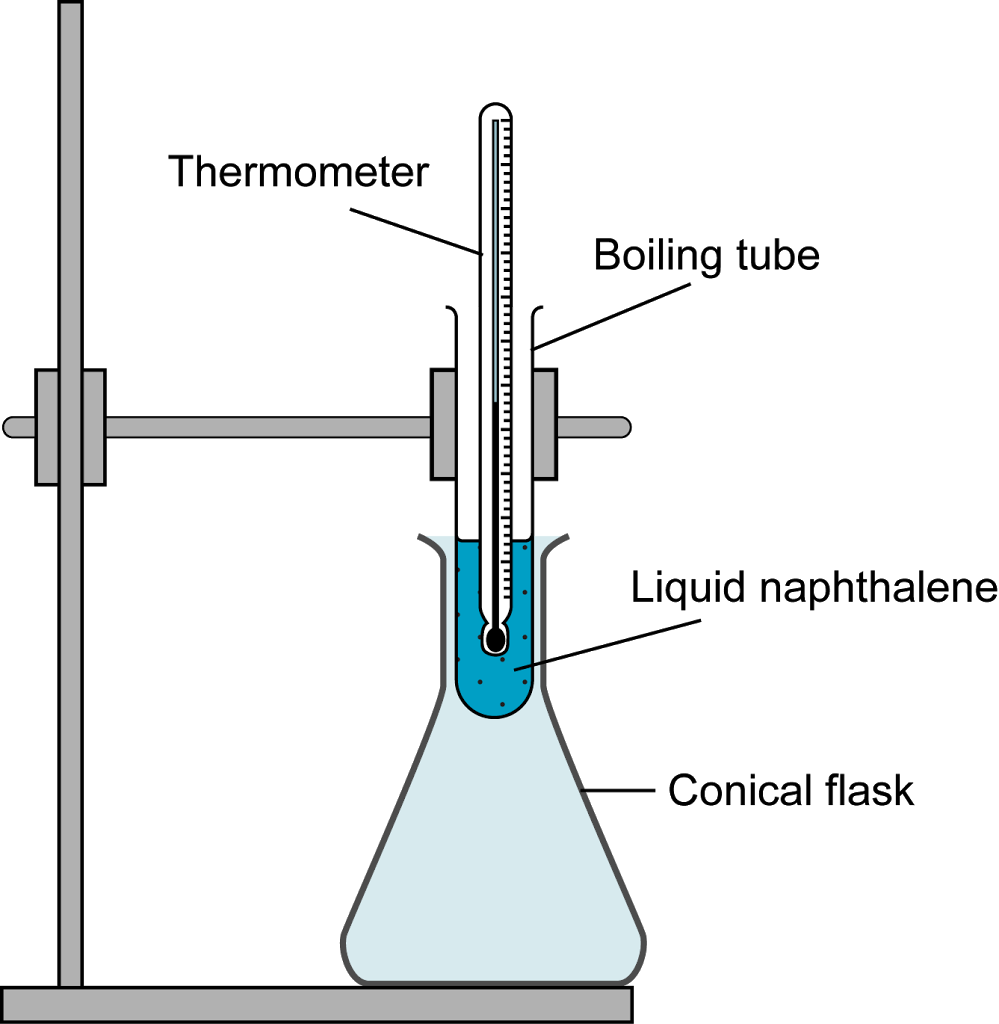
Purpose To determine the freezing point of a known substance, naphthalene II. Observe the change in temperature from 90o to 70o Celsius, recording the temperature at regular intervals, preferably 15 seconds. Mixing salt and ice decreases the freezing temperature of water, lowering the freezing point of the new mixture. What happen to the temperature of naphthalene during melting? Over a time period of 12 minutes and 30 seconds, we recorded the temperature at regular 15 second intervals, and, with this data, constructed a chart showing the general curve. Sublimation is what the process of the dried ice with water being poured on it.
Answered: reezing point of pure naphthalene T1 =…

In this experiment, we record out data on a computer program that allows us to draw a graph to be able to measure its trend. Data Time Elapsed Temperature of Naphthalene Time Temperature Initial 0:00 100oC 7:00 78. Purpose To determine the freezing point of a known substance, naphthalene II. As the temperature rises, the naphthalene will eventually start to melt. Ignite the Bunsen burner and using direct heat melt the naphthalene powder until it completely turns to a liquid. Upon inspection of the graph and our data chart, we found the experimental freezing point of naphthalene to be around 79oC.
What is the melting point and freezing point of naphthalene?
Why temperature is constant during melting and freezing of naphthalene take place? Thinking fast on your feet, and being the the smart chemistry student you are, you and your friends go buy oil, water and dry ice. Assemble the Bunsen burner, attaching one end of the hose to the burner and the other to a Use a clamp to hold the thermometer in place. Purity will then be determined by melting point. When every senior is trying to come up with the perfect prank to play on the school. Considering the time that it takes for the balloons to blow up and the capacity they can hold, you were able to calculate the time in which it took to… Comparing the Coolant Effects of Dry Ice and Ice What we learned in this lab is how to compare the abilities of dry ice and solid water to act as heat absorbers. You will graph the temperature changes before drawing any conclusions.
Artikelen
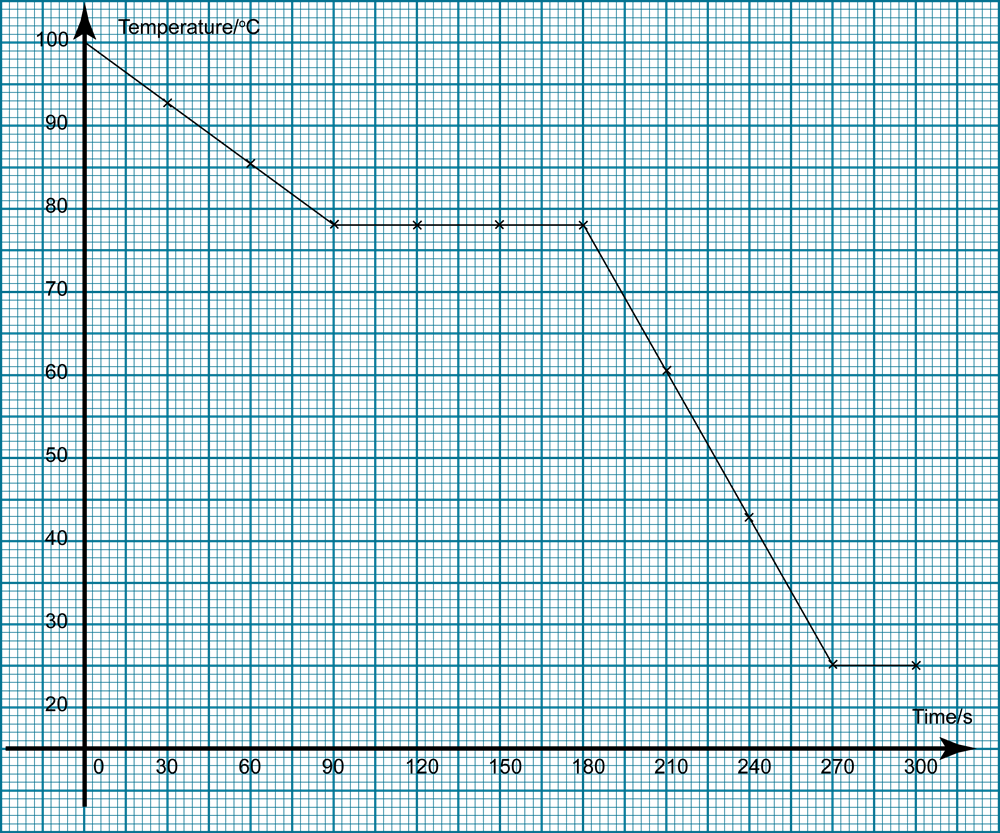
Another fundamental principle that is discussed in this lab is the principle where we relate the freezing point depression of a pure solvent to the molality of the solution. So in this experiment the main topics are chemical compounds, temperature, and evaporation. We also learned to determine which substance was the most cost effective. As with the melting point, increased pressure usually raises the freezing point. We then heated and melted the two substances… Freezing Point Depression Lab The goal of this experiment is to find the molar mass of an unknown substance by measuring the freezing point depression of a known amount in an aqueous solution. The melting point of an organic solid can be determined by introducing a tiny amount into a small capillary tube, attaching this to the stem of a thermometer centred in a heating bath, heating the bath slowly, and observing the temperatures at which melting begins and is complete.