Anhydrous sodium carbonate, also known as soda ash, is a white, odorless, crystalline solid with a formula of Na2CO3. It is a highly alkaline compound, with a pH of around 11.2 in aqueous solution.
Sodium carbonate is a versatile compound with a wide range of applications, including use as a water softener, detergent booster, and pH adjuster. It is also used in the manufacturing of glass, soap, and paper, as well as in the refining of petroleum products.
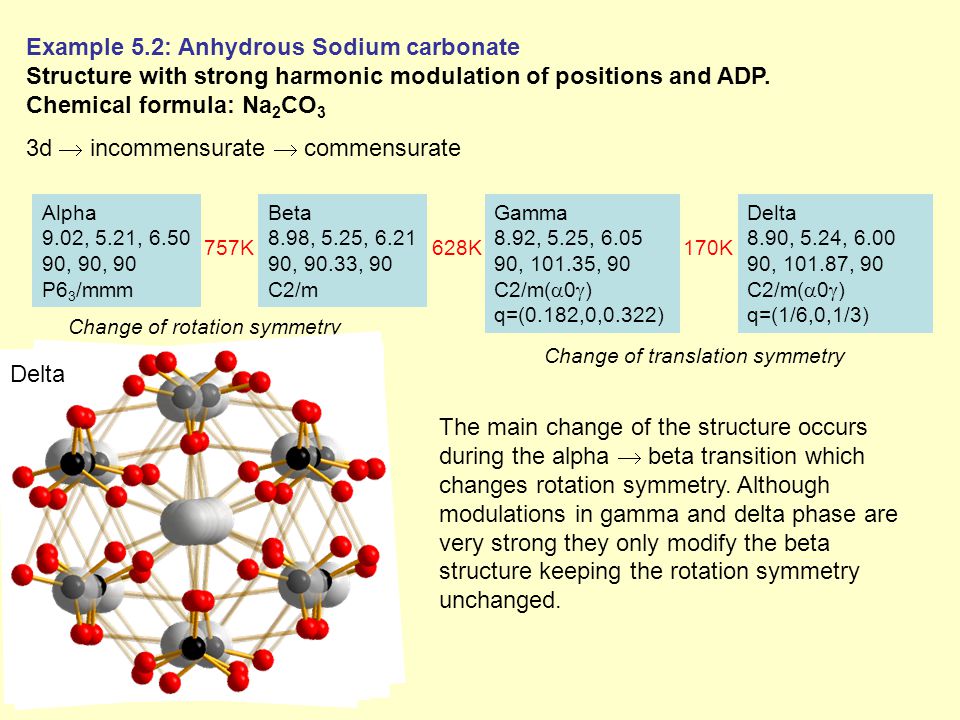
The chemical formula for anhydrous sodium carbonate reflects its molecular structure, which consists of two sodium atoms bonded to a single carbon atom, which is then bonded to three oxygen atoms. The compound is an ionic compound, meaning that it is composed of positively charged ions (sodium) and negatively charged ions (carbonate).
Sodium carbonate can be synthesized through a variety of methods, including the Solvay process and the ammonia-soda process. In the Solvay process, salt, limestone, and ammonia are combined to produce sodium bicarbonate, which is then heated to produce anhydrous sodium carbonate. The ammonia-soda process involves the electrolysis of a sodium chloride solution to produce chlorine and sodium hydroxide, which are then used to produce sodium carbonate.
Anhydrous sodium carbonate is a stable compound under normal conditions, but it will decompose when heated to high temperatures, releasing carbon dioxide gas. It is also soluble in water, forming a strong alkaline solution.
Overall, anhydrous sodium carbonate is an important chemical compound with a wide range of uses in industry and household products. Its formula, Na2CO3, reflects its molecular structure and the arrangement of its atoms, which contribute to its chemical properties and reactivity.