The reaction rate refers to the speed at which a chemical reaction occurs. There are several factors that can affect the reaction rate, including temperature, concentration, surface area, presence of a catalyst, and pressure.
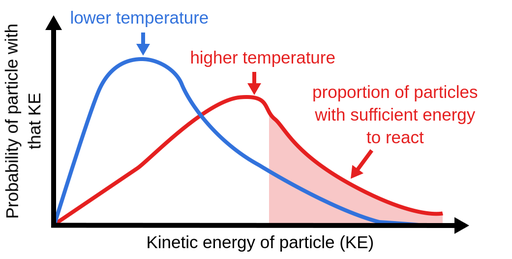
Temperature: Increasing the temperature of a reaction generally increases the reaction rate. This is because higher temperatures lead to an increase in the kinetic energy of the reactant particles, which means they are more likely to collide and react with each other. However, there is a point at which the temperature becomes too high and the reactant particles begin to break apart, resulting in a decrease in the reaction rate.
Concentration: The concentration of reactants in a solution also affects the reaction rate. When the concentration of reactants is high, there are more particles present, which means there is a greater chance for collisions and reactions to occur. On the other hand, when the concentration of reactants is low, there are fewer particles present, which leads to a lower reaction rate.
Surface area: The surface area of a reactant can also affect the reaction rate. When the surface area of a reactant is increased, there is more surface area available for reactant particles to collide and react with each other. This leads to an increase in the reaction rate. For example, if a solid reactant is ground into a powder, the surface area increases and the reaction rate may also increase.
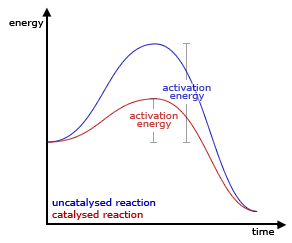
Catalyst: A catalyst is a substance that increases the reaction rate by providing an alternative pathway for the reaction to occur. Catalysts work by lowering the activation energy needed for the reaction to occur. This means that the reactant particles require less energy to collide and react with each other, leading to an increase in the reaction rate.
Pressure: In some reactions, the pressure can also affect the reaction rate. For example, in reactions involving gases, increasing the pressure can lead to an increase in the reaction rate. This is because the increased pressure leads to an increase in the concentration of gas molecules, which increases the chance for collisions and reactions to occur.
In conclusion, the reaction rate is affected by several factors, including temperature, concentration, surface area, presence of a catalyst, and pressure. Understanding these factors can help chemists predict and control the rate of chemical reactions.
:max_bytes(150000):strip_icc()/Chemical-reaction-58ebc1ee3df78c5162aa1992.jpg)