Extraction of sucrose from sugarcane chemistry. Sugar Processing 2022-10-10
Extraction of sucrose from sugarcane chemistry
Rating:
7,8/10
845
reviews
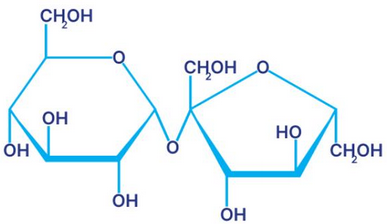
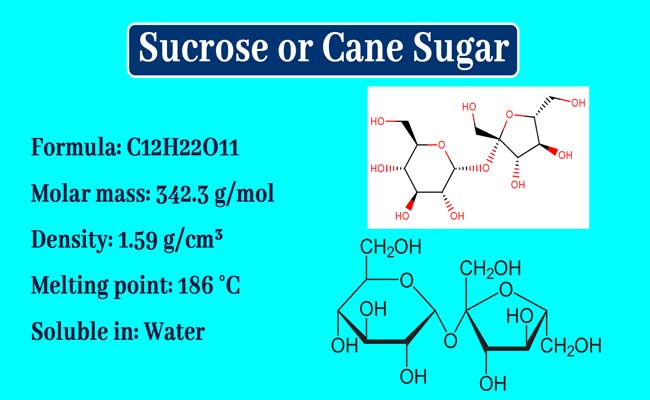
Sucrose, also known as table sugar, is a common sweetener found in many household and commercial products. It is extracted from a variety of sources, including sugar beets and sugarcane. In this essay, we will focus on the extraction of sucrose from sugarcane.
Sugarcane is a tall, tropical grass with a high sugar content. It is grown in warm, humid regions around the world, including Brazil, India, and China. To extract sucrose from sugarcane, the stalks are first harvested and cut into small pieces. These pieces are then crushed to release the juice, which contains sucrose and other sugars.
The next step in the process is to purify the juice. This is done through a series of filtrations and crystallizations, which remove impurities and separate the sucrose from the other sugars. The purified juice is then heated to evaporate the water, leaving a thick syrup known as raw sugar.
Raw sugar is not yet ready for consumption, as it contains a significant amount of impurities and molasses. To further purify it, the raw sugar is subjected to a refining process. This involves adding chemicals such as phosphoric acid and calcium hydroxide to the raw sugar, which helps to remove impurities and create a white, granulated sugar.
Once the refining process is complete, the sucrose is ready for use. It can be used as a sweetener in a variety of products, including baked goods, drinks, and candy.
In conclusion, the extraction of sucrose from sugarcane involves several chemical processes, including filtration, crystallization, and refining. These processes help to purify the sugar and make it ready for use in a variety of products.
US3107605A

Likewise, formic acid, acetic acid, oxalic acie, acrylic acid, chloroethanoic acid, formic anhydride and formyl chloride may also be utilized. Extraction of sucrose 6 steps to making sugar from sugar cane 3 GO! Ketene has also been successfully used as an organic pyrolyzable substance. Preferably, but not necessary to the operation of the process, at least a portion of the raffinate output stream will also be passed to a separation means wherein at least a portion of the desorbent material can be separated to produce a desorbent stream which can be reused in the process and a raffinate product containing a reduced concentration of desorbent material. Carbonation and decolourisation used in refining in India are done simultaneously. Selectivity, B , for an extract component with respect to a raffinate component may be characterized by the ratio of the distance between the center of the extract component peak envelope and the tracer peak envelope or other reference point to the corresponding distance between the center of the raffinate component peak envelope and the tracer peak envelope. Thus the bagasse is suitable for handling on belt conveyors or the like and with minimum further drainage is suitable for feeding into a subsequent mill. This sugary juice is then boiled until thickened and then spun through a centrifuge to remove impurities and coloring from the sucrose.
Next
Extracting the Sucrose Through Sugar Processing
This arrangement ensures a permanent liquid seal of the discharge opening 37 between the rollers 2 and 3 with a minimum quantity of excess maceration liquid. The most prominent use of sucrose is as a food. Accordingly, it is the object of this invention to provide an improved process for the recovery of sucrose from a sugar source containing impurities therein. The aromatic nitrogen compounds of nitrobenzene, 1-ni- tronaphthalene, aminobenzene and 2-amine toluene may also be successfully used as the organic pyrolyzable substance of this invention. The terms "extract product" and "raffinate product" mean products produced by the process containing, respectively, an extract component and a raffinate component in higher concentrations than those found in the extract stream and the raffinate stream.
Next
Extraction of sucrose from sugar cane

Amines such as dimethylamine and ethylmethylamine, nitriles such as acetonitrile and propionitrile, and carbylamines such as ethyl isocyanide may also be used for the organic pyrolyzable substance of this invention. US1265582A - Process for extracting sucrose from sugar-cane. It is expressed in terms of the volume in cubic centimeters of desorbent pumped during this time interval represented by the distance between the peak envelopes. It is to be understood that the operating parameters of the leaching step will vary over a wide range and will be dependent upon a combination of time, temperature, strength of the leaching solution, etc. Expired - Lifetime Application number US4724315A Inventor Andrew Adams Original Assignee Andrew Adams Priority date The priority date is an assumption and is not a legal conclusion.
Next
Chemistry ( extraction of sucrose from sugarcane ) Flashcards

Sucrose and oxygen are the resulting products, and while the oxygen is released from the plant, sucrose is stored in the cane's fluids. However, neither of the procedures results in a complete separation of the sucrose even though high purity can be obtained. Among the heterocyclic compounds, five member ring compounds such as furan, proline, coumarone, thionaphthene, indole, indigo, and carbazole may be. In sugar-cane mill work, the cane'is usually-passed through a crusher previous to being fed to the first millof a train of mills arranged in tandenuand this crusher not only extracts a large proportion of the juice but prepares the cane for themills. Low glycemic sugar composition Similar Documents Publication Publication Date Title AP2511A en 2012-11-21 Treatment of sugar juice AR243333A1 en 1993-08-31 Sweetener compounds with a density ranging from 0. Sugar cane is harvested and taken to a factory where it is crushed to get the juice out. Sucrose is broken down into glucose and absorbed quickly from your small intestines into your blood stream, raising your blood sugar and providing fuel to your cells.
Next
Extraction of sucrose from webapi.bu.edu

The presence of glycosidic bond causes its water decomposition. Ethyl sulfate and sodium laurel sulfate are also appropriate for use. The chamber is contained within a temperature control means and, in addition, pressure control equipment is used to operate the chamber at a constant predetermined pressure. However, due to the slow desorption of sucrose it was not possible to establish a half-width value and consequently a retention volume could not be measured. Posted on October 19th, 2018 by Carrier Vibrating According to the USDA, over 185 million tons of sugar were processed worldwide in 2017, which is a record level of sugar processing. The retention volume for sucrose was calculated by measuring the distance from time zero or the reference point to the midpoint of the sucrose peak and subtracting the distance representing the void volume of the adsorbent which was obtained by measuring the distance from the same reference point to the midpoint of the tracer used to determine the void volume. It is therefore necessary that sucrose be separated from these crystallization inhibitors in order to facilitate the recovery thereof.
Next
EP0047518A2

They are washed to extract any sucrose present and returned to the fields to be used as fertilizer. In the process of the present invention the adsorbent will comprise a shaped replicate of a particle aggregate comprising a carbonaceous pyropolymer containing recurring units of at least carbon and hydrogen atoms while the desorbent material will comprise an aqueous alcoholic solution. In a milling apparatus for extracting sucrose from sugar cane in which sugar cane particles are passed through a mill with a discharge opening to discharge the resulting compressed bagasse into a maceration liquid, comprising a funnel shaped trough of substantially rectangular cross-section arranged adjacent to and across said discharge opening, said discharge opening being completely submerged in said macreation liquid to form an airtight seal for said discharge opening two opposite sides of said trough being formed by side plates of said funnel, the upper and lower sides of said funnel being formed by an upper and a lower plate each having a plurality of orifices therein, said plates being arranged at dillerent angles to the horizontal plane and extending upwards from said opening with the smaller gap between them adjacent said opening, said orifices in said upper and said lower plate being arranged nearest to said opening for the admission of maceration liquid, a grid extending from the upper plate of said funnel in the same plane thereof, a further grid extending from the lower plate in the plane of the lower plate with its outer end forming an overflow edge for said bagasse. The leaching of the base material of the type hereinbefore set forth may be effected over a wide range of temperatures, said range being from about ambient 20° - 25° C. One preferred embodiment of this process utilizes what is known in the art as the simulated moving-bed countercurrent flow system. In the first test the desorbent material consisted of deionized water.
Next
Sugar Processing
Google has not performed a legal analysis and makes no representation as to the accuracy of the date listed. The basic operations taking place in zone 2 are the displacement from the non-selective void volume of the adsorbent of any raffinate material carried into zone 2 by the shifting of adsorbent into this zone and the desorption of any raffinate material adsorbed within the selective pore volume of the adsorbent or ad-- sorbed on the surfaces of the adsorbent particles. Concentration Clarified juice is processed through multiple-effect evaporators — which are often comprised of three to five stages of evaporation to concentrate the juice. Filter them and separate them. Sucrose hydrolyzes to glucose and fructose at a pH less than 7. This precipitate can then be removed from the diluted molasses solution by filtration followed by washing, to remove adhering impurities.
Next
US1265582A
In this zone, the feed stock contacts the adsorbent, an extract component is adsorbed, and a raffinate stream is withdrawn. During its passage through this cell, the heated and limed juice not only takes up sucrose from the shredded'or dis-' integrated cane in this cell, but also fixes impurities in the said shredded or disintegrated cane. As described above visual evidence that the outlet of the mill is properly sealed is obtained by allowing the liquid to also fill the space between the top plate of the trough and roller 2 as indicated by the dotted level line 39. Similarly, alkenes which suffice include ethene, propene, 1-butene, 2-butene and 1-pentene. Like relative volatility, the higher the selectivity the easier the separation is to perform.
Next
How Is Sucrose Exacted From Sugar Cane?

The midpoint of the D 20 peak envelope established the void volume. This solution was subjected to a pulse test in a manner similar to that set forth in Example I. In these tests 70 cc. The final sucrose granules are then packed and shipped out for consumers. Defection: By mixing 2 3% solution of lime in sugarcane juice, the ph value of the solution is reduced to about 7. For this reason, there is no reaction with sucrose tollens reagent, Fehling solution, hydrogen cyanide and phenylhydrazine. Only four of the access lines are active at any one time: the feed input stream, desorbent inlet stream, raffinate outlet stream, and extract outlet stream access lines.
Next






