The enthalpy of neutralization, also known as the heat of neutralization, is the heat that is released or absorbed during a chemical reaction in which an acid and a base react to form a salt and water. This process is known as neutralization, and it is an exothermic reaction, meaning that it releases heat. The enthalpy of neutralization is an important concept in chemistry because it helps to understand the energy changes that occur during chemical reactions, which can have practical applications in a variety of fields, such as chemical engineering and biochemistry.
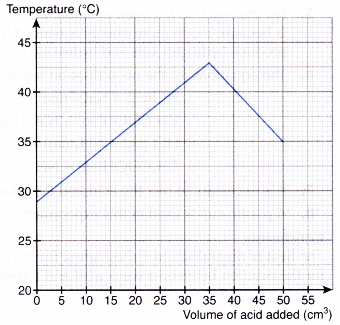
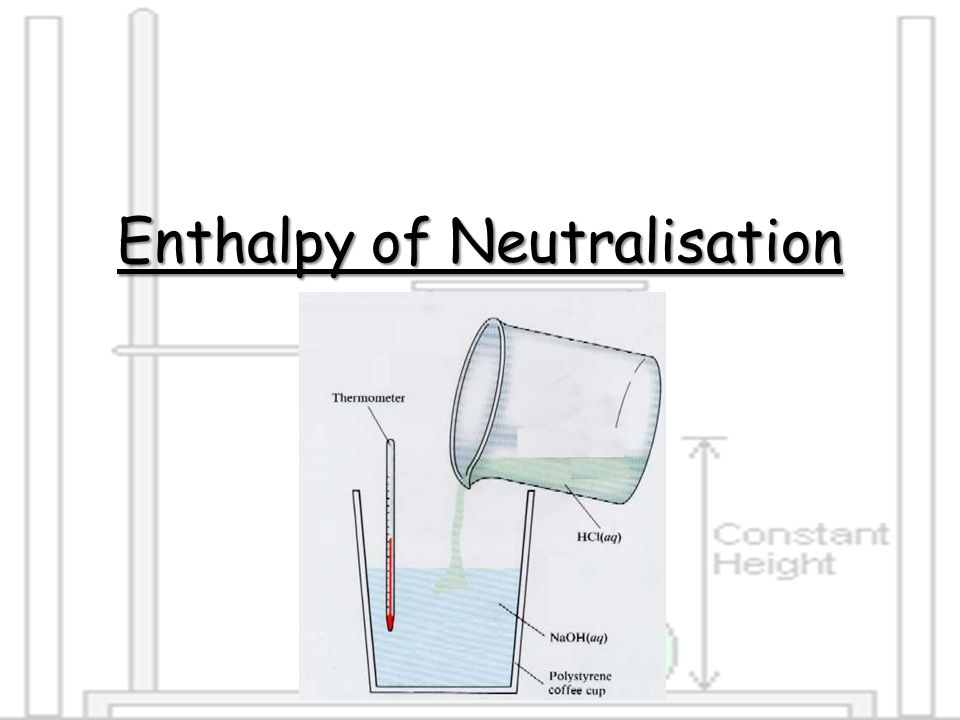
One way to measure the enthalpy of neutralization is through an experiment in which the temperature change of the reacting solution is measured. To do this, a known volume of acid and a known volume of base are mixed together in a calorimeter, which is an insulated container that is designed to minimize the loss of heat to the surrounding environment. The temperature of the reacting solution is measured before and after the reaction, and the enthalpy of neutralization can be calculated using the following equation:
ΔH = (mCΔT) / n
where ΔH is the enthalpy of neutralization, m is the mass of the reacting solution, C is the specific heat capacity of the solution, ΔT is the change in temperature, and n is the number of moles of the reactant that has undergone a chemical reaction.
There are several factors that can affect the enthalpy of neutralization in an experiment. One important factor is the concentration of the acid and base. If the concentrations are high, the enthalpy of neutralization will be greater because there are more molecules present to react and release heat. Another factor is the type of acid and base being used. Different acids and bases have different enthalpies of neutralization, and this can be used to predict the heat release or absorption of a particular reaction.
In conclusion, the enthalpy of neutralization is an important concept in chemistry that helps to understand the energy changes that occur during chemical reactions. By measuring the temperature change of a reacting solution, it is possible to calculate the enthalpy of neutralization using the equation ΔH = (mCΔT) / n. The enthalpy of neutralization can be affected by several factors, including the concentration of the reactants and the type of acid and base being used. Understanding the enthalpy of neutralization can have practical applications in fields such as chemical engineering and biochemistry.