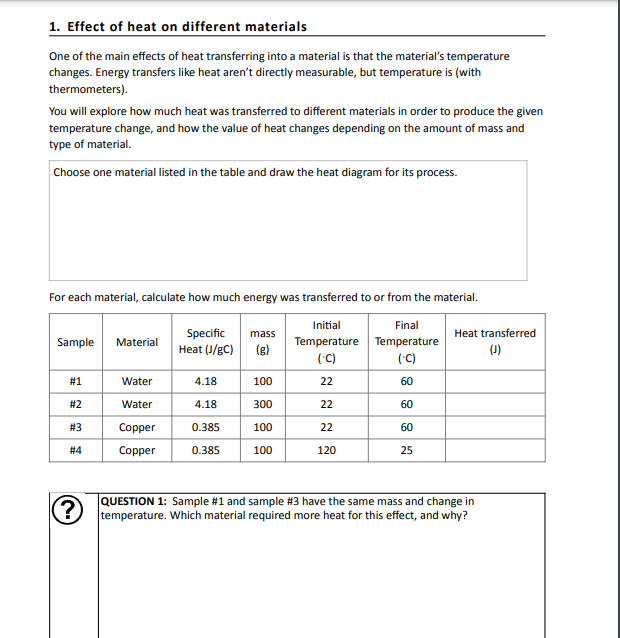
Heat can have a variety of effects on materials, depending on the specific material and the temperature it is subjected to. Some materials may become more brittle or fragile when exposed to high heat, while others may become more flexible or malleable. In some cases, heat can even cause a material to undergo a chemical reaction, changing its properties entirely.
One common effect of heat on materials is a change in their physical properties. For example, metals are known to become softer and more pliable when heated, which is why they can be molded or shaped through processes like forging or casting. At high enough temperatures, metals may even become liquid, allowing them to be poured into molds and cooled into specific shapes. On the other hand, ceramics and other brittle materials may become more fragile when exposed to heat, making them more prone to cracking or breaking.
Heat can also have an effect on the chemical properties of materials. For instance, heating certain materials can cause them to undergo chemical reactions that change their composition. This is often the case with polymers, which are long chains of molecules that can be altered through processes like thermal decomposition. When subjected to high heat, the bonds between these molecules may break, leading to the formation of new compounds and a change in the material's properties.
Heat can also affect the electrical properties of materials. Many materials, such as semiconductors, are sensitive to temperature changes and may exhibit altered electrical conductivity as a result. This can be useful in certain applications, such as temperature-sensitive sensors, but it can also be a drawback in electronic devices that are prone to overheating.
In conclusion, the effects of heat on materials can be complex and varied. Some materials may become more pliable or flexible when exposed to high temperatures, while others may become more brittle or fragile. Heat can also cause materials to undergo chemical reactions, changing their properties and composition. Understanding how heat affects different materials is important for a wide range of applications, from manufacturing and construction to electronics and energy production.
Specific heat capacity

There comes a point where some electrons are released from the atoms that form the gas, but they continue to coexist, both the freed electrons and the atoms, converted into ions. Hardness is the resistance of being scratched or indented by another material. Answer: making the surface of the bag colder, for example by placing ice cubes next to it, will make condensation occur faster. The softening of vibrations is behind one of the many mechanisms of how thermoelectric devices impede the flow of heat. So as well as expanding them, heat can also cause substances to change state.
The Effect of Heat on Rubber

For example, Gas Tungsten Arc Welding or Arc Welding or MIG type process for the same size weld. In crystals, which possess an ordered atomic structure, these waves are called phonons: wave packets of atomic displacements that carry thermal energy equal to their frequency of vibration. For example, when we heat water in a saucepan, the bottom liquid rises and the cooler surface water sinks, heats up, and rises again. As a result, they will eventually break down over time. An object with a wider area has more surface particles working to conduct heat. How does a Thermometer work? Answer: see the images to the right. Answer: solids melt when heated.
Properties of Metals Affected by Welding and Heat
Answer: what if your finger felt cold not because of evaporation, but because the water was cold? For example, you are far more likely to see quick, visible changes in a material like vinyl than ones like concrete or brick. At a higher temperature, the volume they occupy is greater. The flow of thermal energy is transferred from one object to another. Although all matter is different, there are a series of characteristics that allow us to classify it according to its state of aggregation. Celsius Scale Outside the United States, most of the world uses the Celsius scale to measure temperatures. The material between them is in the state of plasma heated by nuclear fusion.
Effect Of Heat Input In Welding: Comprehensive Research Data
Strength, can be altered drastically by welding. This is the case of metals, because this phenomenon occurs at a higher speed than in others. This is because the heat from your body is transferred to the liquid water and carried away in water vapour. The general properties of matter are those that allow us to differentiate some substances from others. Using cutting-edge electron microscopes and novel techniques, a team of researchers at the University of California, Irvine, the Massachusetts Institute of Technology and other institutions has found a way to map phonons — vibrations in crystal lattices — in atomic resolution, enabling deeper understanding of the way heat travels through quantum dots, engineered nanostructures in electronic components.