An acid-base titration is a common laboratory procedure used to determine the concentration of an unknown acid or base solution. It involves the gradual addition of a known concentration of acid or base, called the titrant, to the unknown solution until the reaction between the acid and base is neutralized, resulting in the formation of water and a salt. This point of neutralization is known as the equivalence point and can be identified by the change in pH of the solution as the titrant is added.
In order to perform an acid-base titration, it is important to first prepare the necessary equipment and solutions. This includes a buret, which is a calibrated glass tube used to measure and dispense the titrant, and a pH meter or indicator solution to monitor the pH of the solution. The titrant and unknown solution should also be properly labeled and prepared according to the specific concentration and volume needed for the experiment.
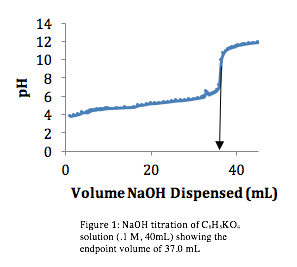
Once the equipment is set up and the solutions are prepared, the titration can begin. The buret is filled with the titrant and the unknown solution is placed in a beaker or flask. The titrant is then slowly added to the unknown solution, a small volume at a time, while continuously stirring and monitoring the pH of the solution. As the titrant is added, the pH of the solution will change until it reaches the equivalence point, where the acid and base are completely neutralized and the pH is neutral (pH 7).
The volume of titrant needed to reach the equivalence point can be used to calculate the concentration of the unknown solution. This is done using the formula: M1V1=M2V2, where M1 and V1 represent the concentration and volume of the titrant, and M2 and V2 represent the concentration and volume of the unknown solution. By solving for M2, the concentration of the unknown solution can be determined.
It is important to accurately measure the volume of titrant used and the pH of the solution at the equivalence point in order to obtain accurate results. Additionally, it is important to carefully follow all safety precautions when handling potentially hazardous chemicals and solutions.
Overall, the acid-base titration is a useful and widely-used laboratory technique that allows for the precise determination of the concentration of an unknown acid or base solution. It is important to follow proper procedure and accurately measure and record all relevant data in order to obtain reliable and accurate results.