Atomic models, or theories, are ways of explaining the structure and behavior of atoms, the basic building blocks of matter. Throughout history, scientists have proposed various atomic models in an attempt to better understand the properties of atoms and how they interact with one another. These models have evolved over time as new experimental evidence has been gathered, leading to a deeper understanding of the nature of atoms and their role in the universe.
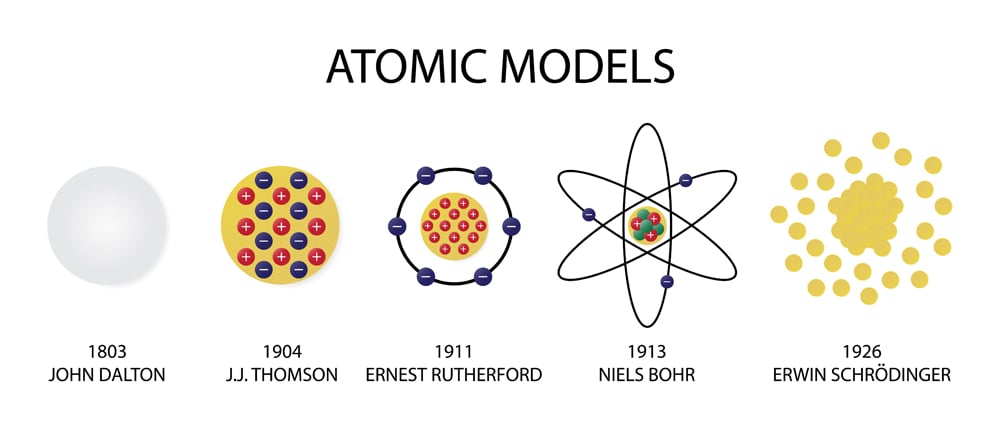
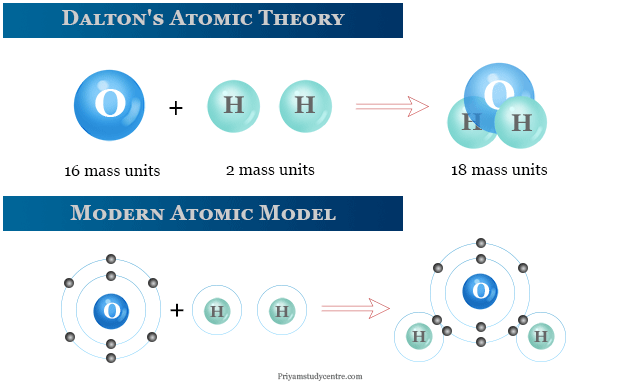
One of the earliest atomic models was proposed by John Dalton in the early 19th century. Dalton's model was based on the idea that atoms are indivisible and indestructible particles that make up all matter. He also suggested that atoms of different elements have different masses, and that the properties of a substance are determined by the type and number of atoms present. This model was able to explain many observations about chemical reactions and the behavior of gases, and it remained widely accepted for many years.
However, Dalton's model faced several challenges, particularly when it came to explaining the behavior of light. In the late 19th century, the discovery of cathode rays and the electron led to the development of a new atomic model known as the "plum pudding" model. This model, proposed by J.J. Thomson, suggested that atoms are composed of a positive charge surrounded by a cloud of negative electrons. This model was able to explain the observed properties of cathode rays, but it did not account for the observed behavior of light, which was eventually explained by the theory of quantum mechanics.
Another significant atomic model was proposed by Niels Bohr in 1913. Bohr's model, which was based on the idea of quantized energy levels, was able to explain the spectral lines observed in the emission of light from atoms. This model also introduced the concept of the electron shell, in which electrons occupy specific energy levels around the nucleus. Bohr's model was able to successfully explain many of the observed properties of atoms, but it did not fully account for the behavior of electrons in atoms and was eventually superseded by more complete models.
One of the most significant atomic models of the 20th century was the "electron cloud" model, which was developed by Erwin Schrödinger and others in the 1920s. This model introduced the concept of wave-particle duality, in which electrons were not thought of as individual particles but as waves that could occupy multiple energy levels at once. This model was able to explain many of the observed properties of atoms and was the basis for the development of quantum mechanics, a theory that has had a profound impact on our understanding of the behavior of matter and energy at the atomic and subatomic scales.
Throughout history, scientists have proposed various atomic models in an effort to better understand the structure and behavior of atoms. These models have evolved over time as new experimental evidence has been gathered, leading to a deeper understanding of the nature of atoms and their role in the universe. Today, our understanding of atoms is based on a combination of several different models and theories, including quantum mechanics, which provides a detailed and accurate description of the behavior of atoms and the fundamental particles they are made up of.