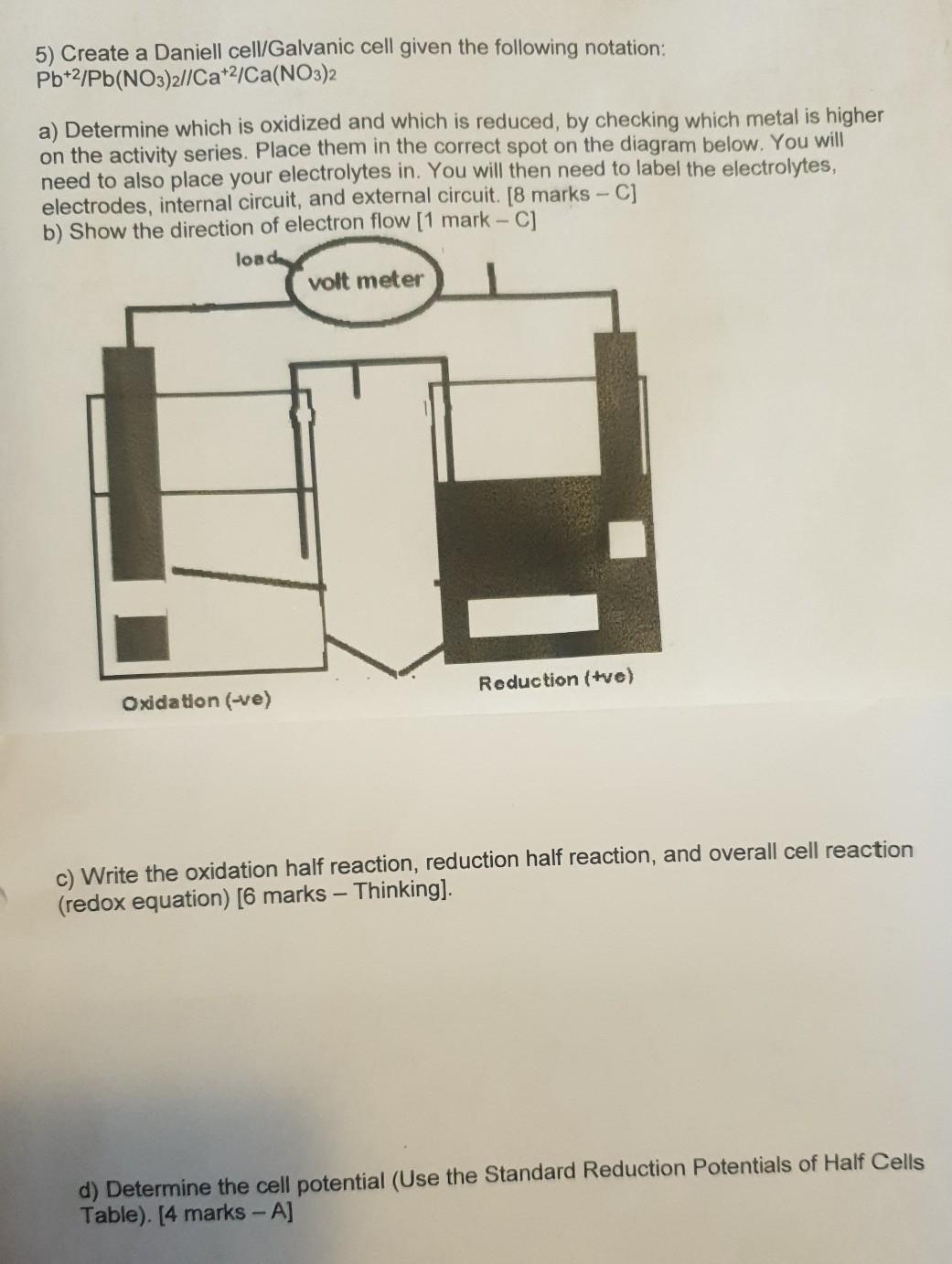
A galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous redox reaction. In other words, it is a device that produces an electric current by using the energy released when one chemical substance is oxidized and another is reduced.
The basic structure of a galvanic cell consists of two half-cells, each containing a different metal and its corresponding salt solution. The two half-cells are connected by an ion-conducting salt bridge, which allows ions to move between the half-cells and maintain electrical neutrality.
The reaction that takes place in a galvanic cell is known as the cell reaction, and it occurs at the electrodes of the cell. The electrode at which the oxidation reaction occurs is called the anode, and the electrode at which the reduction reaction occurs is called the cathode.
The voltage of a galvanic cell is determined by the standard reduction potential of the half-reactions occurring at the electrodes. The standard reduction potential is a measure of the tendency of a half-reaction to occur, and it is related to the energy change of the reaction. A galvanic cell with a higher standard reduction potential will produce a higher voltage.
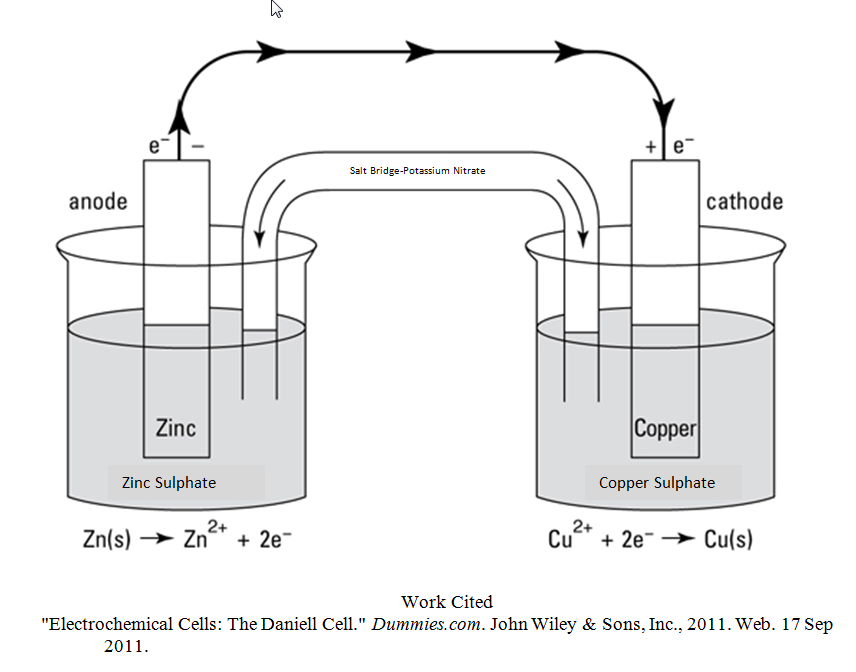
On the other hand, a Daniell cell is a type of galvanic cell that was developed in the 19th century by John Daniell. It is similar to a galvanic cell in that it also converts chemical energy into electrical energy through a spontaneous redox reaction. However, the Daniell cell has some key differences from a galvanic cell.
One of the main differences between a Daniell cell and a galvanic cell is the type of electrolyte used. In a Daniell cell, the electrolyte is a solution of sulfuric acid, whereas in a galvanic cell, the electrolyte is a salt solution. This difference in electrolyte leads to some differences in the cell reaction and in the voltage produced by the cell.
Another difference between a Daniell cell and a galvanic cell is the type of electrodes used. In a Daniell cell, the electrodes are made of copper and zinc, whereas in a galvanic cell, the electrodes can be made of any metal or combination of metals.
Overall, the main difference between a galvanic cell and a Daniell cell is the type of electrolyte and electrodes used. Both types of cells convert chemical energy into electrical energy through a spontaneous redox reaction, but the specific details of the cell reaction and the voltage produced can vary depending on the type of cell.