The copper cycle lab is a common experiment conducted in high school chemistry or college level general chemistry courses. It is designed to demonstrate the principles of oxidation-reduction reactions and the concept of a redox couple. In this lab, a series of reactions take place involving copper metal, copper ions, and various other chemical species. The purpose of this lab report is to summarize the results and observations of the copper cycle lab, and to discuss the underlying chemistry involved in the reactions.
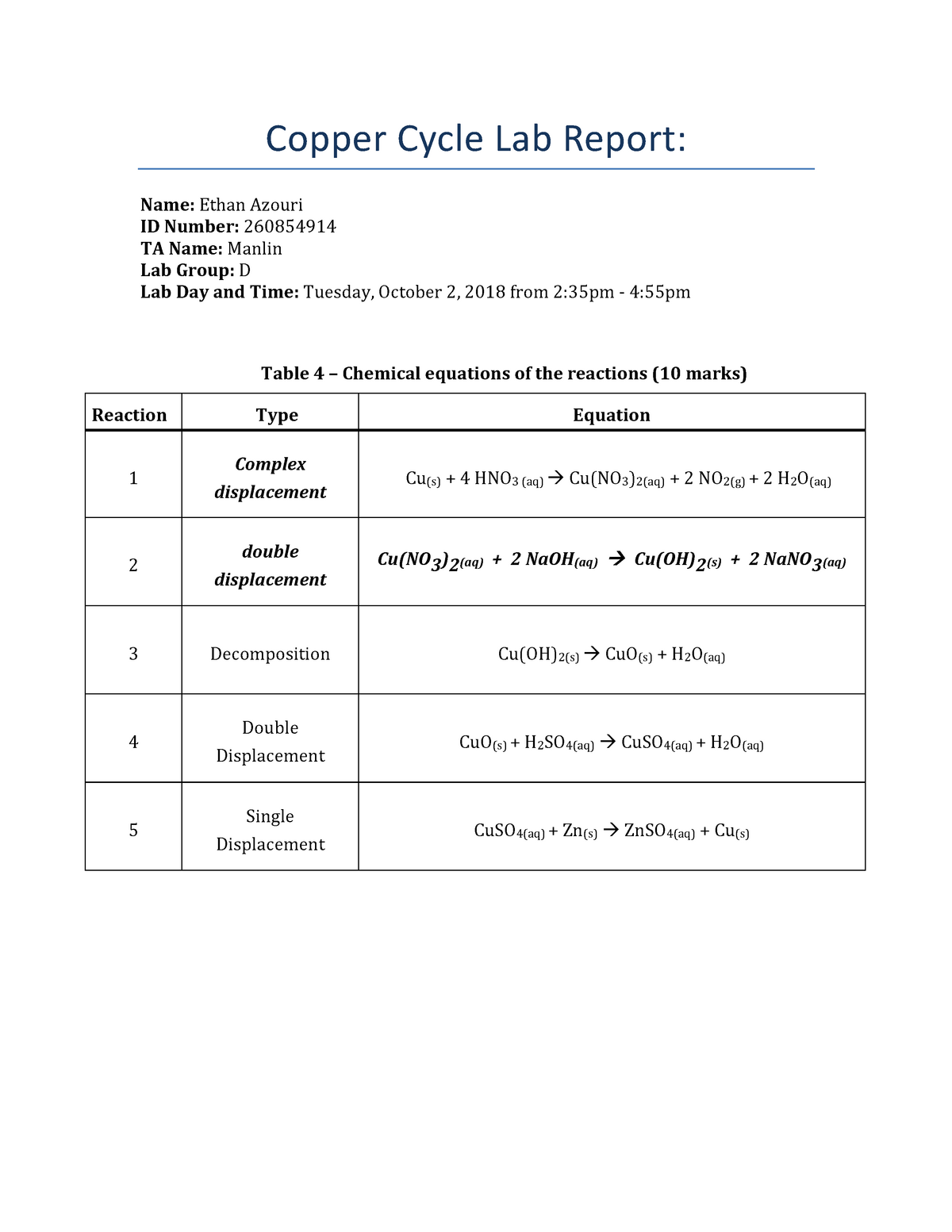
The first step of the lab involves preparing a copper oxide-copper sulfate solution by mixing solid copper oxide with aqueous copper sulfate. When these two chemicals are combined, a redox reaction takes place, in which the copper oxide is reduced to copper metal while the copper sulfate is oxidized to sulfuric acid. The balanced chemical equation for this reaction is:
CuO + CuSO4 -> Cu + H2SO4
The next step involves adding hydrochloric acid to the solution, which causes the copper metal to dissolve, forming copper ions in solution. This reaction can be represented by the following equation:
Cu + 2HCl -> CuCl2 + H2
The copper ions can then be precipitated out of solution by adding sodium hydroxide, which forms solid copper hydroxide:
CuCl2 + 2NaOH -> Cu(OH)2 + 2NaCl
The copper hydroxide can then be heated to form solid copper oxide, completing the cycle:
Cu(OH)2 -> CuO + H2O
Throughout the lab, various observations were made, including the change in color of the solutions and the appearance of solid products. The solutions were initially a blue-green color due to the presence of copper ions, but as the reactions progressed, the solutions changed color as the copper ions were reduced or oxidized. The appearance of solid products, such as copper metal or copper hydroxide, was also noted.
Overall, the copper cycle lab is a useful demonstration of the principles of oxidation-reduction reactions and the concept of a redox couple. It allows students to observe the changes that take place as copper undergoes various redox reactions, and to understand the underlying chemistry involved. By completing this lab, students should have a better understanding of the role of copper in chemical reactions and the factors that can affect its behavior.