Copper is a chemical element with the symbol Cu and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. Copper is found in a variety of minerals in the earth's crust and is the third most abundant element after oxygen and silicon. It has been used by humans for thousands of years, and its unique properties make it an essential element in many modern technologies.
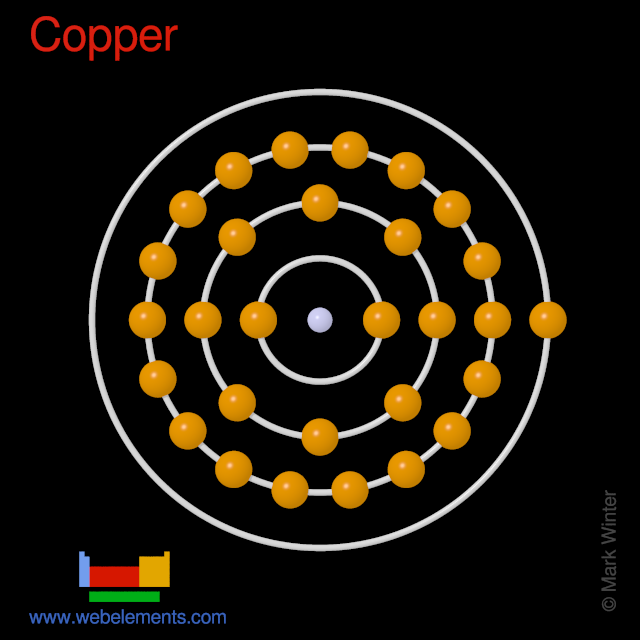
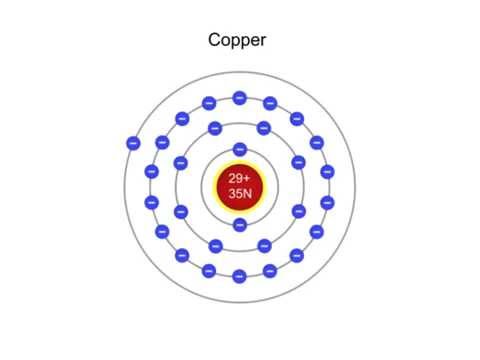
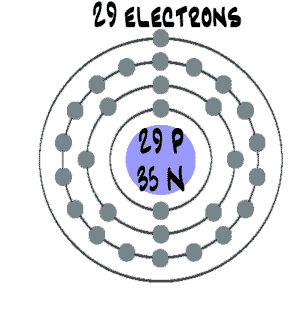
At the atomic level, copper is made up of a central nucleus containing 29 protons and 35 neutrons, surrounded by 29 electrons. The protons and neutrons make up the majority of the mass of the atom, while the electrons are much lighter and are responsible for the chemical properties of the element. The number of protons in the nucleus determines the element's atomic number, which is 29 for copper.
The structure of the copper atom can be modeled using various theoretical models. One such model is the Bohr model, which describes the behavior of the electrons in the atom. According to the Bohr model, the electrons in an atom occupy shells or energy levels around the nucleus. Each shell has a specific energy level, and the electrons in the outermost shell, known as the valence electrons, are responsible for the chemical properties of the atom.
In the case of copper, the electrons occupy the first four shells, with the last shell containing the valence electrons. The first shell can hold a maximum of two electrons, the second shell can hold a maximum of eight electrons, the third shell can hold a maximum of 18 electrons, and the fourth shell can hold a maximum of 32 electrons. Thus, copper has a total of 29 electrons, with the last two electrons occupying the fourth shell.
Another important model used to describe the structure of the copper atom is the quantum mechanical model, which is based on the principles of quantum mechanics. This model takes into account the wave-like nature of particles and is able to provide more accurate predictions about the behavior of atoms and their electrons. According to the quantum mechanical model, the electrons in an atom are not confined to specific energy levels but instead exist as probability clouds around the nucleus.
The properties of copper, such as its high electrical and thermal conductivity, are due to the arrangement of its electrons. Copper has a single valence electron in its outermost shell, which is highly mobile and able to move freely through the metallic lattice. This mobility allows for the easy flow of electrical current through copper, making it an excellent conductor. Copper's high thermal conductivity is due to the ability of its electrons to transfer heat energy effectively.
In summary, the copper atom is a complex structure consisting of a nucleus containing protons and neutrons, surrounded by electrons occupying various energy levels. The arrangement of these electrons determines the unique properties of copper, such as its high electrical and thermal conductivity. Understanding the structure of the copper atom allows us to better understand and utilize this important element in modern technologies.