Bromination of e stilbene lab report. Bromination of (E) 2022-10-16
Bromination of e stilbene lab report
Rating:
6,9/10
243
reviews
Writing a successful complaint letter can be a useful way to resolve a problem or issue that you have encountered. Whether you are complaining about a product or service, poor treatment from a company or individual, or any other issue, a well-written complaint letter can help you get the resolution you are seeking. Here are some tips for writing a successful complaint letter:
Be clear and concise: Be sure to clearly state the problem and what you would like done to resolve it. Avoid rambling or including unnecessary information.
Use a professional tone: While it is understandable to be upset about the issue you are complaining about, it is important to maintain a professional and respectful tone in your letter. Avoid using rude or confrontational language.
Include all relevant details: In order to fully understand the problem and provide a resolution, it is important to include all relevant details in your letter. This includes the date and time the issue occurred, any relevant names or identification numbers, and any other pertinent information.
Keep a copy: It is a good idea to keep a copy of your letter for your own records. This will allow you to refer back to it if necessary and provide proof of your complaint if the issue is not resolved.
Follow up: If you do not receive a response or resolution to your complaint within a reasonable amount of time, it is appropriate to follow up with another letter or phone call to ensure that your complaint has been received and is being addressed.
By following these tips, you can effectively communicate your issue and increase the chances of a successful resolution. Remember to be patient and persistent, as it may take some time to resolve the issue.
Prelab Bromonation of E Stillbene

The investigations contained: dehydrohalogenation, bromination and corrosive catalyzed hydration. Mayo, Dana, Ranold Pike, and David Forbes. Weigh the product and determine the yield. Microscale Organic Laboratory with Multistep and Multiscale Synthesis. In order to calculate the percent yield, the theoretical yield is taken. To determine this, the value of the constant was found at different concentrations of HIn, HIn-, and at varying pH, which was used to determine the concentration of H+.
Next
Addition of Bromine to trans
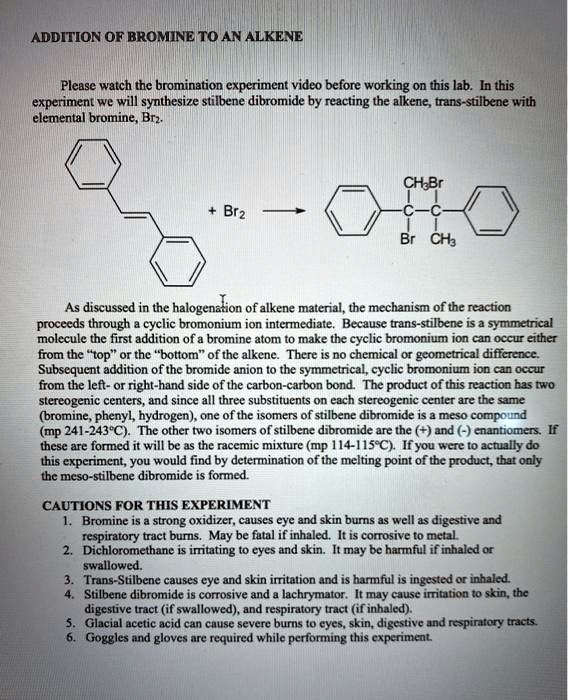
The improper temperatures were most likely the main cause for the low percent yield. Stopper the reaction flask loosely and stir the mixture for 15 min. After the reaction occurred the results were analyzed by IR and by an ignition test. Post -Lab Questions: 2. To start off, in an Erlenmeyer flask approximately 0.
Next
Bromination of E stilbene

Nageswer Rao, and V. Then they were added dropwise to the solution causing the reaction to occur this was evident by the change in color of the solution. The hue of bromine, which was initially brownish red, quickly changes to clear with the formation of a double bond. In contrast to the formation of meso-stilbene dibromide as the major productin the electrophilic addition of bromine to trans-stilbene in nonpolar solvents,stereoselectivity decreases in cyclodextrin cavities and a significant yield of dl-stilbene dibromide is also obtained. The melting point was determined. This low yield can be explained from a poor recrystallization technique combined with potential contamination.
Next
Bromination of e
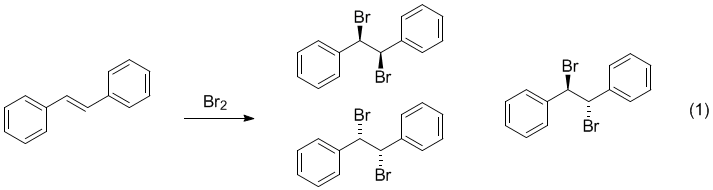
The reaction of bromine with alkenes is an addition reaction where the nucleophilic double bond attacks the electrophilic bromine Mayo, et. All experimental values of K for this trial fell within the range. This results in trans dibromide. While the method produces the higher-melting meso compound as the end result, one will ascertain this. The temperature was too low for the reaction to occur completely and effectively. The mixture was cooled before collecting the product by a vacuum filtration.
Next
bromination of stilbene essay

The percent yield was determined by taking the mass of the final ketone and dividing it by the original mass of the alcohol. Additionally, the bromination of E- stilbene will be carried out, and its anti-addition status will be evaluated as it produces a meso compound with a higher melting point. Some modifications were made. The range then that all experimental values of K must fall under is 3. Thus, the product was indeed …show more content… In this experiment, it was possible to produce the major products from bromination of acetanilide and aniline.
Next
(PDF) Bromination ReportSheet Fall

Coordination with methanol is unnecessary as it reduces its nucleophilicity and makes less protons available to coordinate with the carboxylic acid. In addition, the melting point of the product was measured to be 164. In light of this, bromine can discriminate between unsaturated compounds with straight chains or rings. Alkenes are natural exacerbates that have a carbon-carbon twofold bond as the useful gathering for that compound. The values of K for solutions 1-5 and U were 4.
Next
Bromination lab report

The E-Stilbene reacted with pyridinium hydromide perbromide to form the pure 1R, 2S -stilbene dibromide. Bromination is a type of electrophilic aromatic substitution reaction where one hydrogen atom of benzene or benzene derivative is replaced by bromine due to an electrophilic attack on the benzene ring. The percent yield was calculated to be 88. This is possible because there are two paths a reaction can take to be successful. When Benzaldehyde reacted with the Wittig reagent, it produced two products: E-Stilbene and Z-Stilbene. The theoretical yield was 0 g, and the percent yield was calculated to be 98%.
Next
Stilbene Lab Report
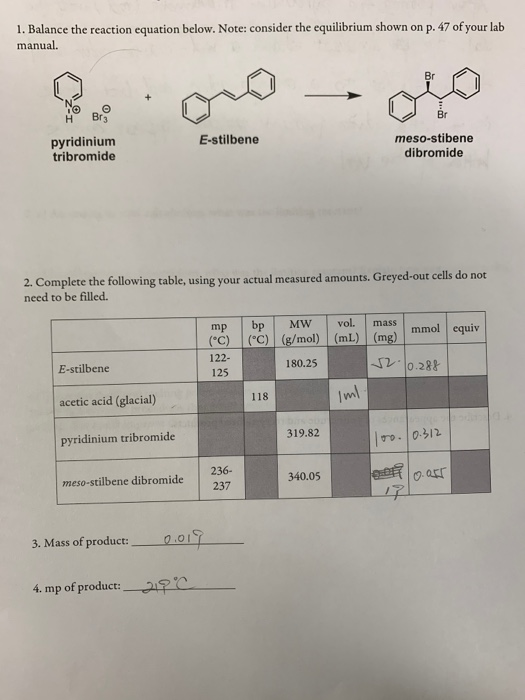
The FTIR Greener Brominations Essay Greener Brominations Abstract In this experiment, trans-stilbene was brominated and debrominated with the use of three methods: bromination with pyridinium tribromide, bromination with hydrogen peroxide and hydrobromic acid, and debromination with zinc. The percentage yield was calculated to 46. It is considered an addition reaction in which bromine is added to an alkene. E-Stilbene is a trans isomer of methylene, that can follow two mechanistic pathways when conducting the addition reaction of bromination. The difference in the two possible outcomes is the intermediate formed during the reaction, before the product is made. This reversal of stereoselectivity isattributed to the polar environment provided by the secondary hydroxyl groupsof cyclodextrins which stabilises the acylic α-halocarbonium ion intermediateand also hinders the approach of the incoming tribromide ion. Then stir it for 10-15 minutes.
Next
exp 6 webapi.bu.edu

Bromination of E -Stilbene — Report Written by: Katie Banas Reference: Experimental Organic Chemistry - A Miniscale and Microscale Approach, Sixth Edition, Gilbert and Martin Introduction This experiment was designed to compare and contrast the various intermediates formed during the bromination of an alkene. The experiment continued regardless, and the pyridium bromide dibromide was also heated and dissolved at 85ºC. Conclusion: It was found that E -stilbene could be brominated in order to synthesize the second intermediate in a line of reactions so that meso-stilbene could be obtained. Although the percent yield was low the experiment did produce meso-stilbene dibromide. According to the data and calculations the initial mass of stilbene was 0 and the final mass was. Stir or swirl the mixture to effect dissolution Bromination and Isolation 1.
Next








