A bath bomb is a small, spherical or cylindrical-shaped product that is designed to be dropped into a bath tub filled with water. It contains a mixture of chemicals that react with the water to create a fizzing, effervescent effect, as well as releasing fragrances, colors, and other additives.
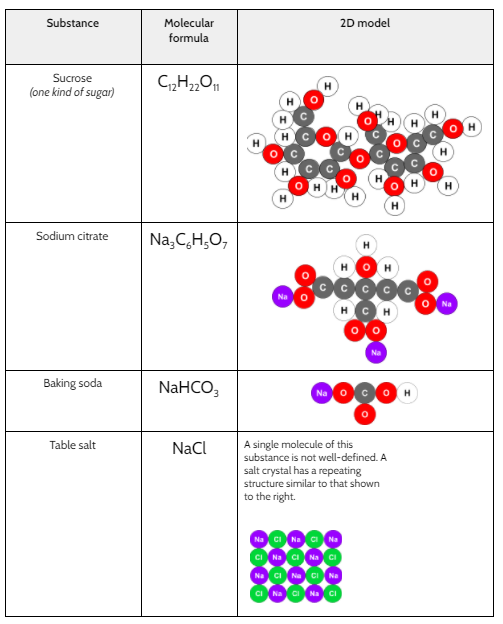
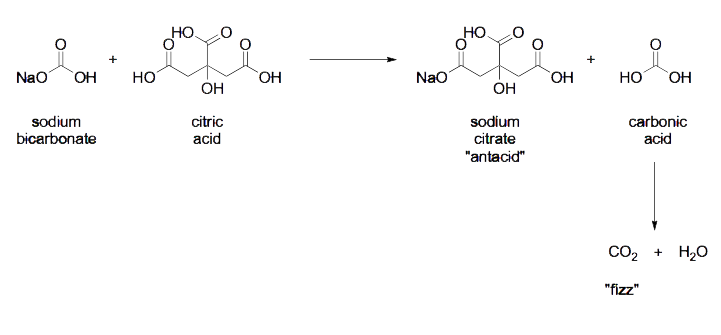
The main chemical reactions that occur when a bath bomb is dropped into water are the release of carbon dioxide gas and the neutralization of an acid with a base. The carbon dioxide gas is produced through the reaction of an acid and a carbonate or bicarbonate. The acid used in most bath bombs is citric acid, which is a weak acid commonly found in citrus fruits. The carbonate or bicarbonate that reacts with the citric acid to produce carbon dioxide gas is typically sodium bicarbonate, also known as baking soda.
The reaction can be represented by the following chemical equation:
Citric acid + Sodium bicarbonate → Sodium citrate + Carbon dioxide + Water
In addition to producing carbon dioxide gas, the reaction between the acid and the carbonate or bicarbonate also results in the neutralization of the acid, which means that the pH of the water is raised from acidic to neutral or slightly basic. This neutralization reaction can be represented by the following chemical equation:
Citric acid + Sodium bicarbonate → Sodium citrate + Water
In addition to citric acid and sodium bicarbonate, bath bombs also contain a variety of other ingredients that contribute to their fizzing, fragrant, and colorful effects. These may include essential oils, fragrances, dyes, and other additives.
While bath bombs can be a fun and relaxing addition to a bath, it is important to be aware of the potential risks associated with using them. Some people may have allergies or sensitivities to certain ingredients in bath bombs, and the chemicals in bath bombs can also be harsh on the skin if used excessively. It is recommended to follow the instructions on the bath bomb packaging and to use them in moderation.
Chemical Reactions

Potassium bitartrate, also known as cream of tartar, acts as a binding agent that keeps the entire mixture together. When you mix the water, vegetable oil, fragrance, and food coloring together in the third bowl, it should look similar to this one depending on what color food coloring you are using. I buy The citric acid and the sodium bicarbonate combine with water to form sodium citrate and carbon dioxide and water. Conclusion The findings of this investigation are that the appropriate amounts of chemicals needed to create the perfect bath bomb are, 5 grams of bicarbonate soda and 3 grams of citric acid. Also, keeping moisture out makes them last longer, and you can do this using silica gels and airtight storage containers.
How do bath bombs work?
What makes bath bombs Fizz? Then, that positively charged hydrogen from the citric acid and the negatively charged bicarbonate from the baking soda mingle, very quickly undergoing a series of reactions. Materials scientists and engineers develop materials, like metals, ceramics, polymers, and composites, that other engineers need for their designs. The amount of each ingredient is also important. Then pop them out. Important: Part of the challenge of making bath bombs is adding the right amount of wet ingredients. Add the same amount each time you fill the bowl. This type of reaction usually produces salts and liberates gases.
Bath Bomb History

The heart of every bath bomb recipe is simple chemical reaction: when sodium bicarbonate baking soda is combined with an acid usually citric acid in an aqueous solution i. If you add more oil or leave the water spritz out, you will have a denser product that will bubble more when it comes in contact with water. The water molecules in the air are enough to create carbon dioxide gases, rendering the bath bomb a dud. Stay tuned for part two where we will be presenting a sample bath bomb recipe and demonstrate how to mold bath bombs using different molds. Is a bath bomb an exothermic reaction? Simple household ingredients, many of which can be bought in the local supermarket, along with easily sourced Can I Make Bath Bombs at Home? Also, getting water in the eyes may cause irritation and should be avoided. Extra Cornstarch Recipe 2tbsp. How do you think having more or less filler will affect how fizzy the bath bombs will be and how quickly they can dissolve in the tub? You can immediately move on to the next section in the Procedure, Testing the Bath Bombs, or you can store the bath bombs in sealed plastic bags until you are ready to test them.