Alcohol density refers to the amount of mass contained in a given volume of an alcoholic beverage. Water density, on the other hand, refers to the amount of mass contained in a given volume of water. It is important to understand the density of both alcohol and water, as this can affect the overall composition and characteristics of a mixture containing both substances.
One of the key differences between alcohol and water is their densities. Alcohol is generally less dense than water, meaning that it is less heavy and occupies a larger volume for the same mass. For example, the density of ethanol (the type of alcohol found in most alcoholic beverages) is 0.789 grams per milliliter, while the density of water is about 1 gram per milliliter. This means that if you have equal volumes of alcohol and water, the water will be heavier and more dense.
This difference in density can affect the way that mixtures of alcohol and water behave. For example, if you mix alcohol and water together, the alcohol will tend to float to the top because it is less dense. This can be seen in cocktails or other mixed drinks, where the alcohol is often added last and floats on top of the other ingredients.
Another important factor to consider when mixing alcohol and water is the fact that alcohol has a lower boiling point than water. This means that when alcohol is heated, it will evaporate more quickly than water. This can be useful in the production of alcoholic beverages, as it allows for the separation of the alcohol from the water through distillation.
In summary, alcohol density and water density are important properties to consider when mixing the two substances. Alcohol is generally less dense and has a lower boiling point than water, which can affect the way that mixtures of the two behave. Understanding these properties can be useful in the production and consumption of alcoholic beverages.
physical chemistry

Expected result It will sink. Keep away from heat, sparks, open flames, and hot surfaces. The main reason is hydrogen bonding. If you continue to use the Website, you are consenting to our use of Cookies for the time being. Water is essentially a tasteless, odorless substance that is available in liquid form under conditions of standard pressure and temperature. On the other hand most of the alcohols are consumed as hard drinks, lounge drinks or beverages on occasional purposes.
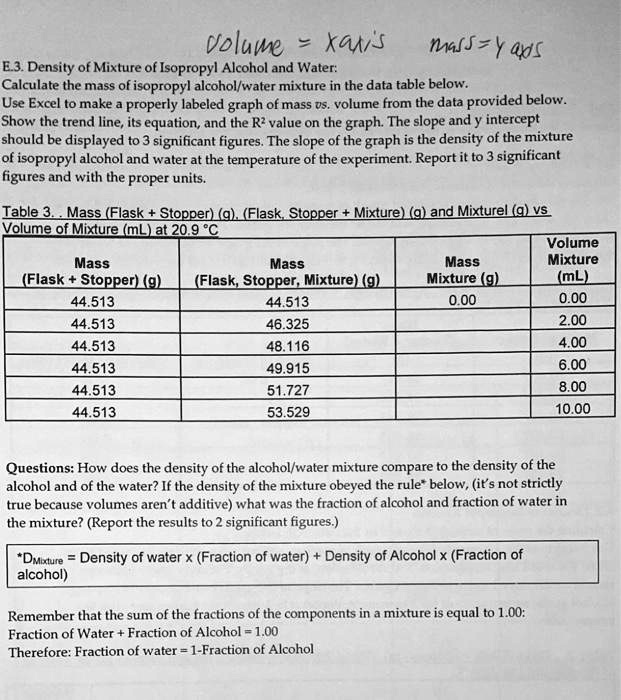
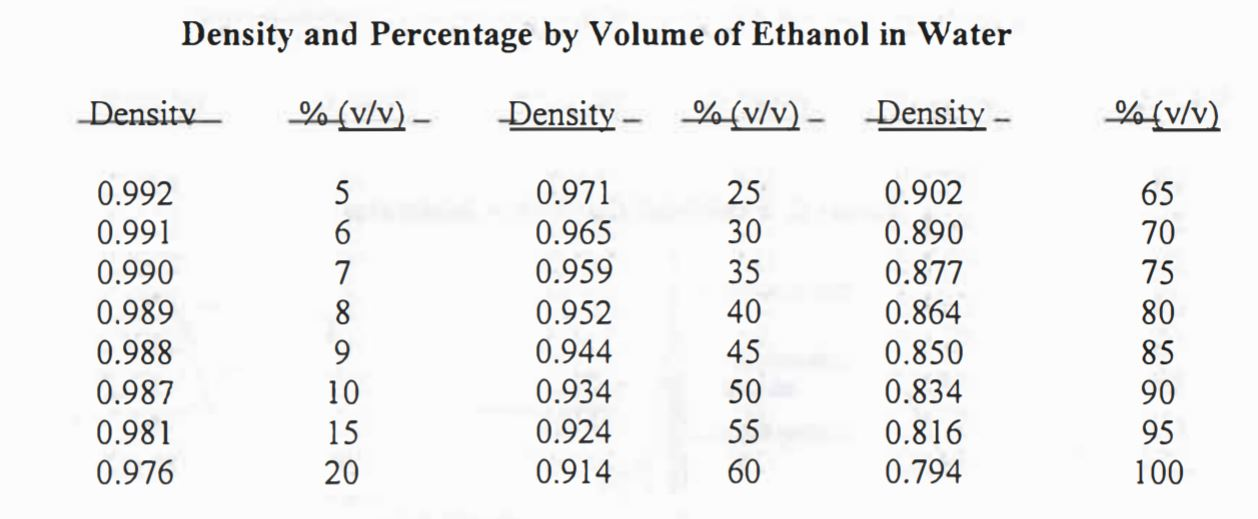
Density and Concentration Calculator for Mixtures of Isopropyl Alcohol and Water

The search on this page works by searching the content of each page individually, much like any web search. We may use these Cookies to provide you with services such as watching a video or adding user comments. Have students compare equal volumes of water and vegetable oil and test whether the oil floats or sinks when added to water. We don't collect information from our users. With these compounds it is the exceptionally strong polarity of water that gives it its dissolving power. Well organized Distilleries will want an instrument created log of precise temperature, latest calibration, % Alcohol by volume, Proof, batch number, product name, and date. We also saw that water is far less effective as a solvent for nonpolar covalent compounds such as oil.
Density of Aqueous Solutions of Organic Substances as Sugars and Alcohols
It also exists in two other forms, as Talking of the chemical composition of water, a single molecule of water comprises of one oxygen atom covalently bonded with two hydrogen atoms, the chemical formula being H2O. If the ethanol density is known in any of these units AlcoDens can be used to determine the strength of the ethanol-water mixture over the range of temperatures from -20°C to 100°C -4°F to 212°F. This lab hand—out may be attached if you do not wish to re—copy the data tables. State the coordinates of the points used. When a detergent molecule contacts a nonpolar compound such as oil, it slides its nonpolar end between the nonpolar molecules of the oil Fig. Exhibiting signs of irritability and extreme mood swings. A complete list of every document table in which the compound occurs is listed, and are hyperlinked to the relevant document table.
Alcohol Concentration to Determine Alcohol by Volume and Alcohol Proof

Dry the outside of the cylinder. Well, that sort of answer requires the object to present a roughly-constant cross-section, which you have not explicitly stated. Besides containing alcohol and water, many alcoholic beverages also have various amounts of extract substances, aroma, and color components, etc. When less accurate methods are used it results in time-consuming trial and error methods being used to achieve the correct blend strength. Water is tasteless while most alcohols have a slight stringent taste Cite APA 7 ,.