Acetylation of aniline with acetic acid is a chemical reaction that involves the substitution of a hydrogen atom in the amino group of aniline with an acetyl group. This reaction can be carried out using a variety of methods, but the most common method involves the use of glacial acetic acid and an aniline derivative as the reactants. The reaction is typically carried out at elevated temperatures and in the presence of a catalyst, such as sulfuric acid or p-toluenesulfonic acid.
The acetylation of aniline with acetic acid is an important chemical reaction that has a wide range of applications. One of the main uses of acetylated aniline is as a precursor to a variety of polymers, including polyacrylonitrile, which is used in the production of synthetic fibers and plastics. In addition, acetylated aniline is also used in the production of dyes, pharmaceuticals, and other chemicals.
The reaction between aniline and acetic acid is typically carried out in a two-step process. In the first step, aniline is converted to its corresponding acetanilide derivative by reacting it with acetic anhydride in the presence of a catalyst. In the second step, the acetanilide derivative is hydrolyzed to yield acetylated aniline.
The acetylation of aniline with acetic acid is a reversible reaction, meaning that it can be reversed by adding a proton to the acetyl group. This can be accomplished by treating the acetylated aniline with a strong acid, such as hydrochloric acid or sulfuric acid.
There are several factors that can affect the yield of the acetylation reaction, including the concentration of the reactants, the type and amount of catalyst used, and the temperature and duration of the reaction. It is important to carefully control these factors in order to obtain a high yield of acetylated aniline.
In conclusion, the acetylation of aniline with acetic acid is a widely used chemical reaction that has a variety of applications in the production of polymers, dyes, pharmaceuticals, and other chemicals. By carefully controlling the reaction conditions, it is possible to achieve high yields of acetylated aniline, which is an important starting material for a wide range of industrial processes.
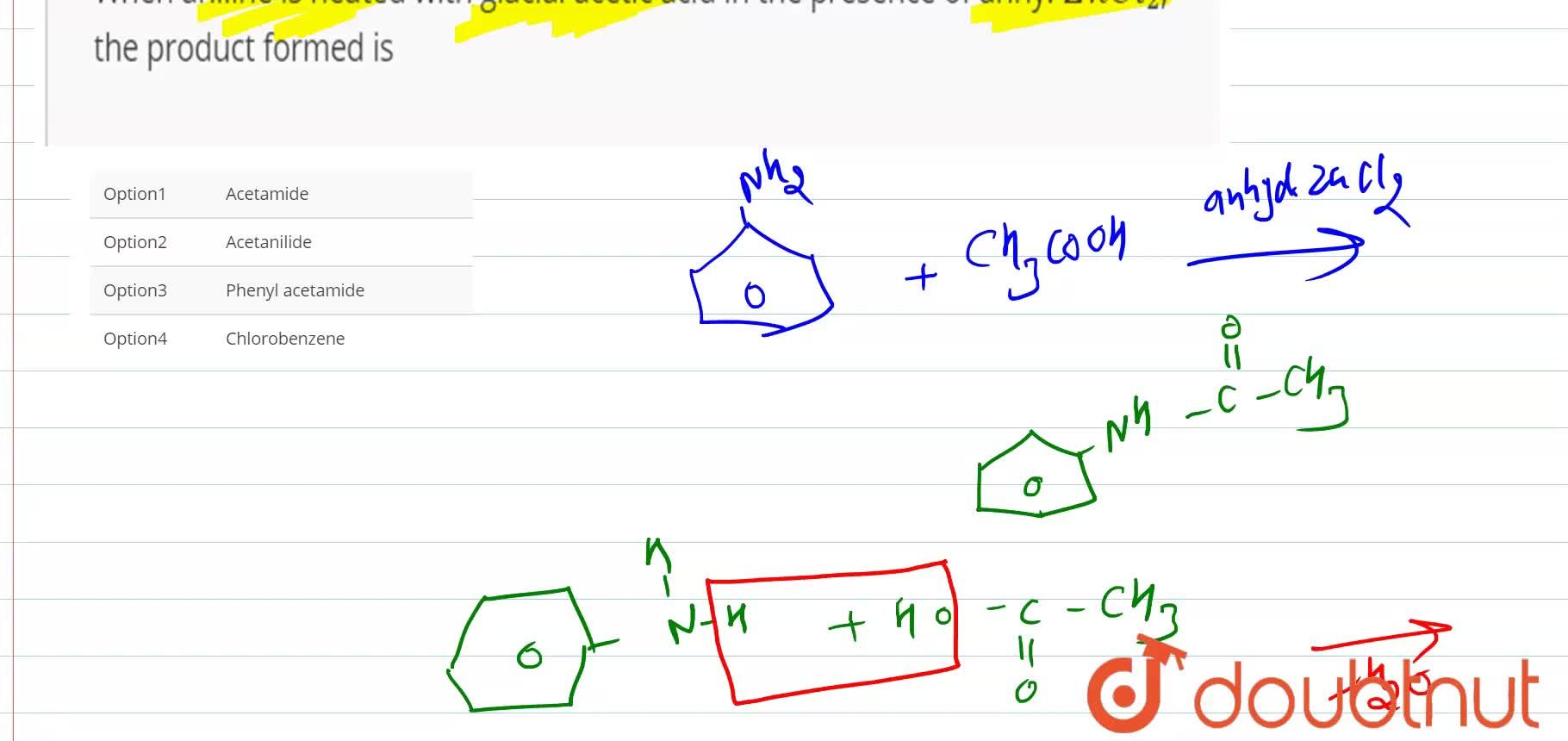
Does aniline react with acetic acid?

How does aniline act as a source of acyl group? It has a role as a protic solvent, a food acidity regulator, an antimicrobial food preservative and a Daphnia magna metabolite. Upon treatment with a standard base, it converts to metal acetate and water. For this purpose after completion of the reaction 4-chloroaniline + ethyl acetate + SSA , CH 2Cl 2 was added to the reaction mixture. NAME: Jamyang Dorjee TA NAME: Shubhendu Karandikar 1. How did the 2 reactions differ? Thus, there is still a demand to develop new and mild methods for the acetylation in the presence of inexpensive and bench top reagents. The stir bar was then turned off, and the mixture was allowed to stand for 5 minutes. Why is direct nitration of aniline not preferred? In addition, deactivated substrates could also be acetylated rapidly Table Acknowledgment The financial support for this work by the Bu-Ali Sina University, Hamedan, Iran is gratefully acknowledged.
Why acetic acid is used in acetylation of aniline?
Introduction The protection of hydroxyl groups of alcohols and phenols is often necessary during the course of various transformations in a synthetic sequence, especially in the construction of polyfunctional molecules such as nucleosides, carbohydrates, steroids, and natural products. Cool the solution in an ice bath and collect the solid acetanilide by vacuum filtration. Which amino acids undergo acetylation? The chemical shifts are reported in ppm relative to the TMS as internal standard, and J values are given in Hz. We can also say that in aniline the lone pair of nitrogen atoms is in conjugation with the πelectrons of the benzene ring and thus takes part in resonance. Acetic acid ethanoic acid and hydrochloric acid…. To find out the efficiency of acylating agents, 4-chloroaniline was chosen as a representative electron-deficient aniline and treated with various acylating agents such as ethyl acetate, acetic acid, vinyl acetate, acetyl chloride, and Ac 2O in the presence of SSA 0.
Synthesis of acetanilide from aniline

Is product obtained alkylation of aniline? The amine will be converted into its acetamide analog also using the acetylation procedure described below. Is glacial acetic acid harmful? Table 1-1 Amine bp °C mp °C Acetamide mp °C aniline 184 -- 114 N-methylaniline 196 -- 102 ortho-toluidine 200 -- 110 meta-toluidine 203 -- 65 2-chloroaniline 209 -- 87 2-ethylaniline 210 -- 111 2,5-dimethylaniline 213 14 139 2,6-dimethulaniline 215 11 177 4-ethylaniline 216 -6 94 2,4-dimethlaniline 217 -- 133 3,5-dimethylaniline 220 10 144 2,3-dimethylaniline 221 4 135 3-chloroaniline 230 -- 72, 78 meta-anisidine 251 -- 81 para-toluidine 200 44 147, 153 3,4-dimethylaniline 224 49 99 2,5-dichloroaniline 251 50 132 para-anisidine 240 58 127 para-bromoaniline 245 66 168 para-chloroaniline 232 72 172, 179. Reactions do not take place with less than 0. Now, we have got a complete detailed explanation and answer for everyone, who is interested! Acetylation is often used to place an acetyl protecting group on primary or secondary amines to reduce their reactivity toward oxidizing agents or electrophiles. The nitric acid used in the nitration step is a fairly strongly oxidizing agent and we could form an N-oxide.