Water is a chemical compound with the molecular formula H2O, which is made up of two hydrogen atoms bonded to one oxygen atom. It is a common substance found on Earth and is essential for all known forms of life. Water has a number of unique properties that make it vital for living organisms and important for a variety of industrial and scientific applications.
One of the most notable properties of water is its ability to exist in three different states: solid, liquid, and gas. Water freezes at a temperature of 0 degrees Celsius (32 degrees Fahrenheit) and boils at a temperature of 100 degrees Celsius (212 degrees Fahrenheit). This is because the molecules of water are held together by a type of chemical bond called a hydrogen bond, which is weaker than the covalent bonds found in most other substances. As a result, water has a relatively high boiling point and a low freezing point.
Another important property of water is its high specific heat capacity. This means that it takes a lot of heat energy to raise the temperature of water, and it also releases a lot of heat when it cools down. This makes water an excellent medium for regulating temperature and maintaining a stable environment. For example, when a person's body becomes too hot, sweat is produced and evaporates, releasing heat and helping to cool the body. Similarly, when water evaporates from the surface of a plant, it cools the plant and helps to regulate its temperature.
Finally, water is a good solvent, which means that it can dissolve many different types of substances. This property is important for a variety of biological processes, as it allows nutrients and other substances to be transported throughout the body. Water is also a universal solvent in the laboratory, meaning that it can dissolve a wide range of chemicals and is often used to prepare solutions.
In conclusion, water is a chemical compound with a number of unique properties that make it essential for life and important for a variety of industrial and scientific applications. Its ability to exist in three different states, high specific heat capacity, and ability to dissolve a wide range of substances are all important characteristics of this versatile compound.
Chapter 3

A loss of just 4% of total body water leads to dehydration, and a loss of 15% can be fatal. Park Ridge, New Jersey: Noyes Publications. When steam comes in contact with a person's skin, that person feels the energy the steam has taken in as a result of breaking up the hydrogen bonds as well as the heat of the boiling water. Visually, water fills cells to help maintain shape and structure Figure 2. Each molecule is bent, with the negatively charged oxygen on one side and the pair of positive-charged hydrogen molecules on the other side of the molecule.
9 Properties of Water along with Examples
How water gets its unique properties? How are the properties of water significant to living organisms? Water Polarity Water molecules are what is called polar. In addition to the evidence from nature, we can also prove it ourselves, namely by pouring a glass of water on sloping land, then the motion of the water will go to the lowest land. Cohesion due to hydrogen bonding contributes to the transport of water against gravity in plants. When water begins to boil, its hydrogen bonds start to break. But the only difference is that in this water, quinine is dissolved. Similar to polarity, some molecules are made of ions, or oppositely charged particles.
What Are the Unique Properties of Water?

The high heat of vaporization means evaporating water has a significant cooling effect. This gives water unique properties, such as a relatively high boiling point, high specific heat, cohesion, adhesion and density. Retrieved August 3, 2016. All these airways will be exposed to water and the portion of water that spreads is the same. The fact that water molecules naturally want to cling to one another means that water has cohesion.
What are the 4 major properties of water? [Ultimate Guide!]

Your heart is constantly working, pumping about 2,000 gallons of blood a day. Water is, of course, not as viscous as honey, but it does have viscosity. As water cools, it becomes denser until it gets very close to its freezing point. At the zero-pressure limit, the compressibility reaches a minimum of 4. Then what is meant by capillarity itself? The liquid is also very identified with water. The Journal of Chemical Physics.
Water Properties and Facts You Should Know

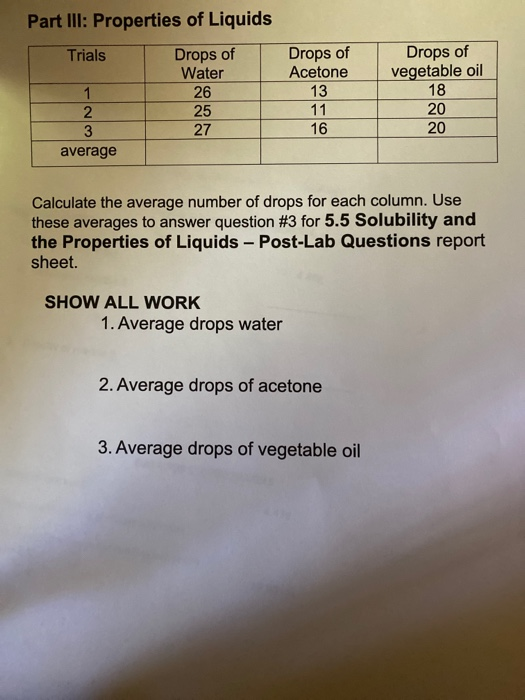
Complete each activity, then answer the questions. This is true for naturally occurring saltwater, such as what is found in oceans, gulfs and other bodies of water, as well as manmade saltwater. The high heat of vaporization refers to the amount of heat energy that we need in order to be able to change one gram of water into gas. High Specific Heat Since water molecules form hydrogen bonds with each other, a lot of energy is needed to break those bonds. United States of America: USGS. Thus all of us can take water for free, even though there are times when water can also be a rare item, namely when the dry season arrives.